Introduction
The symptoms of recurrent cystitis can be triggered by inflammatory or infective causes. Bladder pain syndrome (BPS) and bacterial recurrent lower urinary tract infection can both present with symptoms of recurrent cystitis and cause significant morbidity in affected individuals. These disorders are more common in women, with a general prevalence of around 0.3% and 3% for BPS and UTI respectively.
BPS is defined as a combination of chronic pelvic pain or discomfort, and at least one lower urinary tract symptom, such as urinary urgency or frequency, with symptoms persisting for more than six months; patients commonly express that their pain is worse when the bladder is full. First-line management of BPS is to address pain, implement conservative management with psychological support, modification of diet and fluids, and pelvic floor muscle relaxation. Most patients require combination therapy to optimise symptoms, and additional therapies that can be introduced include oral analgesic drugs, amitriptyline, anticholinergics, and antihistamines (hydroxyzine or cimetidine).
Recurrent urinary tract infections (rUTIs) stem from re-infection or persistence of urinary pathogens in the bladder, and are defined as more than two infections in a six-month period or more than three infections in a 12-month period. In the UK, microbiology laboratories report a bacterial UTI after demonstrating >10⁵ colony forming units (cfu) per ml of urine of a specific type of bacteria. However, bacterial growth of >10³cfu per ml is considered to be clinically important in symptomatic patients. Maintaining a low threshold of suspicion is therefore essential, along with the need to be vigilant for the diagnosis in patients with a relevant clinical history but a reported negative urine culture result. First-line therapy for rUTIs comprises treating any reversible causes, lifestyle advice and supplementary treatments (cranberry tablets, D-mannose). Antimicrobials that are used include antibiotics and / or methenamine. Antibiotics may be taken as an on-demand course or as low-dose daily prophylaxis.
In both the above conditions, it is reasonable to consider intravesical instillations where symptoms are not fully controlled, which include intravesical glycosaminoglycan (GAG) replenishment therapy. In this review we wish to examine the role of intravesical GAG agents which can provide a further option if conservative and first-line therapies fail.
Intravesical glycosaminoglycan instillations
The GAG layer of the bladder was initially identified as a mucus layer on the surface of the uroepithelium which was responsible for the barrier mechanism of the bladder. It comprises a hyaluronic acid (HA) stem with branches of sulphated polysaccharide GAGs including chondroitin sulphate (CS), heparin sulphate and dermatan sulphate. Early work elucidating the role of the GAG layer demonstrated that rabbit bladders deficient in GAGs had a significantly higher infection rate compared to bladders with intact GAG layers [1]. To date, the GAG layer is postulated to have an important protective function, and damage to this layer is widely held as one of the underlying mechanisms for recurrent cystitis in adults.
One of the protective functions of the GAG layer is to prevent the adhesion of pathogenic bacteria to the bladder epithelium, a key step which contributes to their virulence in rUTIs. In addition, it is thought that defects in the permeability barrier function of the GAG layer of the uroepithelium facilitates the entry of urinary irritants, particularly potassium, which trigger the pain and bladder hypersensitivity characteristic of BPS. It has been shown that this GAG layer is defective in individuals with BPS, particularly if there is a lack of chondroitin sulphate in the GAG layer of these patients. A similar mechanism has been postulated for other conditions such as the overactive bladder, radiation cystitis and chronic prostatitis.
Repairing or replenishing the GAG layer consequently addresses the driving factors for disease pathogenesis and may achieve symptomatic relief. Seminal research by Parsons in 1982 [1], demonstrating that exogenous synthetic GAGs could reduce urinary infection rates in damaged rabbit bladders to baseline levels, drove the development of intravesical GAG replacement therapies. These preparations aim to provide the building blocks of the natural GAG layer of the bladder to achieve restoration of its protective function.
Available intravesical GAG instillations include HA-only options (Cystistat® and Hyacyst®), CS-only instillations (Gepan® and Uracyst®), HA with CS (iAluRil®), or Parsons’ cocktail, which comprises heparin in combination with bicarbonate and lignocaine. Pentosan polysulphate sodium (PPS) is a synthetic polysulphated xylan which is thought to be structurally similar to the native GAGs lining the bladder, but it is not actually present in the GAG layer of the bladder. It is available as an intravesical instillation or as an oral formulation (Elmiron®). In the latter preparation, it is thought to replenish the uroepithelium while it is being excreted through the urinary tract.
In spite of GAG replenishment therapy having been used for over two decades with extensive clinical experience and various commercial variants available, the evidence of efficacy or a rationalisation of treatment based on the different GAG replacements has not been fully established. There are only a few controlled studies on GAG replenishment but plenty of uncontrolled trials. We therefore present the evidence available for the role of GAG analogues, alone and in combination, for the treatment of recurrent cystitis.
Efficacy of GAG replacement therapies
The rationale underlying the use of CS instillations is that it is the most abundantly sulphated and often-deficient component of the GAG layer. In Europe, it is available in two different concentrations of 0.2% (40ml) and 2.0% (20ml). In an uncontrolled prospective study of patients with BPS, it was found that 60% of patients responded to CS instillations, which produced a statistically significant improvement in symptom scores from baseline and no significant adverse effects [2]. In a further randomised placebo-controlled trial (RCT), it was reported that twice as many BPS patients had a clinical benefit with intravesical CS compared to those that were given the vehicle control; however, this improvement was not statistically significant [3]. Given that only 65 patients were trialled in the latter study, further well-powered studies are required to elucidate the efficacy of CS instillations.
HA has remained in the market since 1996, and instillations have also demonstrated efficacy in case series of BPS and rUTIs, with a rather broad range of symptom improvement in BPS reported to be between 30% and 85%. One prospective study using HA-only intravesical instillations for the treatment of BPS found significant clinical benefit at a five-year follow-up, with 74% improvement in pain scores from baseline and 50% of patients achieved complete remission [4]. Bladder HA instillations have also been found to be effective in rUTIs, significantly reducing the number of UTI recurrences and the median time to UTI recurrence compared to baseline [5]. Despite the promising results in prospective case series, two RCTs of intravesical HA treatment in BPS failed to show statistically significant improvement of HA treatment over placebo; as these studies remain unpublished in peer-reviewed literature, the true efficacy of HA instillations is under debate [6].
PPS is recommended by the European Association of Urology (EAU) guidelines for the management of chronic pelvic pain, but is currently only for off-label use only in the UK. A meta-analysis of four RCTs that compared PPS with placebo found evidence that PPS may, to a certain extent, improve pain, urgency, frequency in BPS; positive findings varied across the individual studies as the methods and primary endpoints were inconsistent [7]. A more recent double-blind RCT comparing oral PPS with placebo found no statistically significant difference on the Interstitial Cystitis Symptom Index (ICSI), a validated symptom-based questionnaire for BPS [8]. It is known that only 6% of an oral dose of PPS is excreted by the urinary tract [9]. Without an RCT of intravesical PPS to prove its efficacy, it is unclear if the failure of oral PPS treatment was due to a lack of efficacy of PPS or was confounded by its low bioavailability.
HA-CS instillations have the best evidence base so far for their clinical use as they have demonstrated efficacy in rat models of BPS and UTIs, as well as in clinical RCTs. In a rat UTI model, HA-CS installations were shown to result in a significant reduction of bacterial growth rates in urinary and bladder tissue cultures compared to controls. This correlated with an increased thickness of the uroepithelium on histology, suggestive of reduced uroepithelial denudation [10]. In an RCT of 57 women with rUTIs, HA-CS instillations were shown to significantly reduce the rate of UTIs as compared to placebo (86.6% reduction versus 9.6% respectively) at a 12-month follow-up [11]. HA-CS therapy has also been shown to confer a 47% improvement in subjective symptom scores over placebo in patients with rUTIs [11], and improved patients’ Pain Urgency Frequency (PUF) score by 32% from baseline in a prospective study of patients with BPS [12].
It is difficult to pinpoint the underlying reasons as to why HA-CS preparations have achieved superior outcomes over placebo while PPS, HA- and CS-only preparations have not; this may reflect limitations in our understanding of the precise role of the GAG layer and the mechanisms of GAG replacement therapy products. Studies using fluorescence microscopy and radiolabelling have demonstrated that exogenous GAGs bind preferentially to damaged mouse uroepithelium and can restore bladder impermeability to irritant molecules [13], although such findings have yet to be corroborated through biopsies in humans. Nevertheless, this does not discount the known clinical efficacy of GAG replacement therapies. When taken in conjunction with the findings in animal models, the clinical data are consistent with exogenous GAGs having a physical effect on restoring the integrity of the uroepithelium.
Furthermore, it must be noted that many studies on these therapies have not been sufficiently well-powered, and testing heterogeneous patient populations with inconsistent experimental variables precludes fair comparison between intravesical GAG preparations. For example, limited patient numbers, different concentrations of GAG preparations, variable regimens and outcome measures could have contributed to significant discrepancies across studies. In view of these shortcomings, multicentre and international collaboration would be essential to design well-powered studies in the future. Consensus on treatment regimens and standardised outcome measures, such as the use of the ICSI when evaluating subjective symptoms in BPS, would also be of significant help to drive this field of research forward.
Treatment regimens
There is no optimal regimen that has been conclusively identified for intravesical instillations. In general, patients that are started on intravesical GAG replacement undergo induction therapy with one instillation per week for four to six weeks. If there is a positive response to the therapy, maintenance therapy would then be started with fortnightly- or monthly-instillations for up to six months. The various brands of intravesical instillations differ in their recommended treatment course and the evidence base for the use of such dosing regimens is lacking. A prospective study attempted to address this gap in knowledge by assessing the effect of different treatment plans on therapeutic outcomes [14]. Patients were randomised into two groups, with one group receiving nine HA-only instillations given weekly and then monthly, while the other group received 12 instillations given fortnightly. No significant difference in the quality of life index or symptom scores was found for both groups, although there was a significant improvement in urinary frequency in the group of patients with 12 instillations given fortnightly [14]. It is difficult to distinguish if the improvement in urinary frequency was a statistical phenomenon or was due to differences in the treatment regimen (spacing of instillations or the sheer quantity of instillations). Further well-controlled comparative studies will be required to derive an optimal treatment regimen.
Comparison with antibiotic therapy
Oral antibiotics or prophylactic antibiotics are currently standard treatment in the management of rUTIs. The widespread prescription of antibiotics in rUTIs has raised concern regarding the development of antibiotic resistance, particularly when prescribed frequently or without microbiological confirmation of infection. Intravesical GAG replacements can be used in conjunction with antibiotics or as substitutes as they have been reported to be equivalent, if not superior, to the use of prophylactic antibiotic therapies for rUTIs. As evidence, a retrospective study found that HA-CS instillations were equal to antimicrobial and conservative treatments in terms of the total number of UTI recurrences and the median time to the first UTI recurrence following treatment; notably, the risk of UTI recurrence was found to be reduced by 49% in the group of patients who had HA-CS instillations relative to those on standard antibiotic therapy [15].
HA-CS instillations have also been shown in an RCT to significantly reduce the incidence of UTIs at six months’ follow-up relative to prophylactic trimethoprim-sulfamethoxazole [16]. In terms of their safety profile, intravesical GAG instillations are well-tolerated and there are few, if any, side-effects. Most reported cases of side-effects are related to the mode of administration via catheterisation which may cause urethral or bladder discomfort, and a low risk of infection. Thus, the use of intravesical instillations as an alternative or a substitute for prophylactic antibiotics in the treatment of rUTIs is safe, evidence-based, and minimises the risk of antibiotic resistance. More randomised controlled comparative studies of other intravesical agents, which have also shown benefit in patients with rUTIs, should be conducted against antibiotic therapies.
Addition of local anaesthetics for short-term symptomatic relief in BPS
GAG replacement given with local anaesthetics as intravesical ‘cocktails’ for symptomatic relief of pain has been gaining popularity in the treatment of BPS. There are several such ‘cocktail’ formulations in use, such as the ones devised and popularised by Parsons, Whitmore, Bade, Moldwin, Mishra, and Hanno. Alkalinised lidocaine (AL) therapy has been shown by a double-blind RCT to significantly improve patients’ Global Response Assessment (GRA) scores compared to placebo, suggesting efficacy of local anaesthetics as standalone therapy [17]. A subsequent study suggested that AL standalone therapy offered only short-term symptomatic relief, whereas combining AL with GAG replacement may provide a synergistic effect [18].
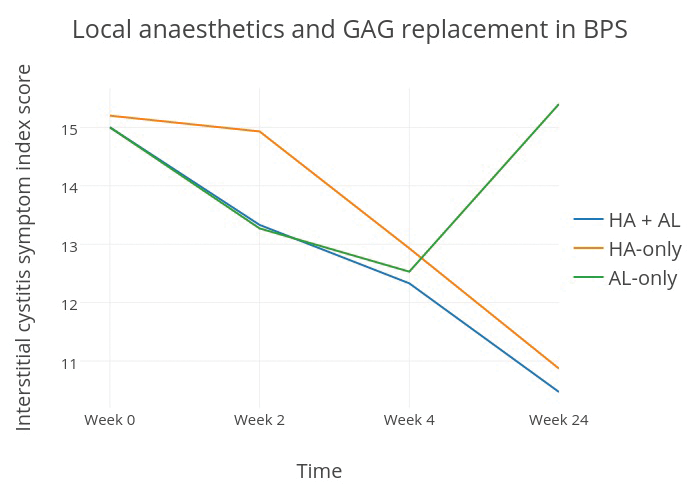
Figure 1: Comparison of the therapeutic efficacy of AL and HA preparations over time (data taken from Lv et al. 2012 [18]).
The study divided BPS patients into three groups: a trial group given HA with AL, as well as two control groups given AL-only and HA-only treatment. At two weeks’ follow-up the two groups that had AL instillations had significant improvement in their symptom scores over the HA-only group; in contrast, the therapeutic effects of HA only became apparent and comparable to that of AL by the fourth week. At 24 weeks’ follow-up, the early therapeutic benefit of AL was abrogated, with 13/15 of the patients under AL-only treatment reported to have recurrence of their disease; by comparison, patients that had HA instillations showed significant improvement from baseline which was sustained for up to 48 weeks (Figure 1). These results are in favour of the combined use of local anaesthetics with GAG replacement therapies, for the fast-onset short-term symptomatic benefits of the former, and the slower-onset but sustained disease-modifying properties of the latter.
Parsons’ cocktail is an example of an in-use GAG replacement mixture which contains 2% alkalinised lidocaine, 40,000IU of heparin, and 8.4% sodium bicarbonate. In a study of patients treated with heparin and AL, 90% (n=32) were found to have rated ‘slight improvement’ or ‘better’ scores on the GRA one month after the treatment regimen; 16.7% of these patients maintained this improvement six months after stopping the treatment regimen [19]. Heparin monotherapy has also been found to improve symptom scores from baseline and 82.5% of patients (n=40), that initially had positive potassium sensitivity tests prior to treatment, tested negative after treatment [20]. Taken together, these studies suggest that the heparin-AL combination in Parson’s cocktail confers clinical benefits that last beyond the local anaesthetic activity of lidocaine and may achieve this effect through the restoration of the heparin component of the GAG layer.
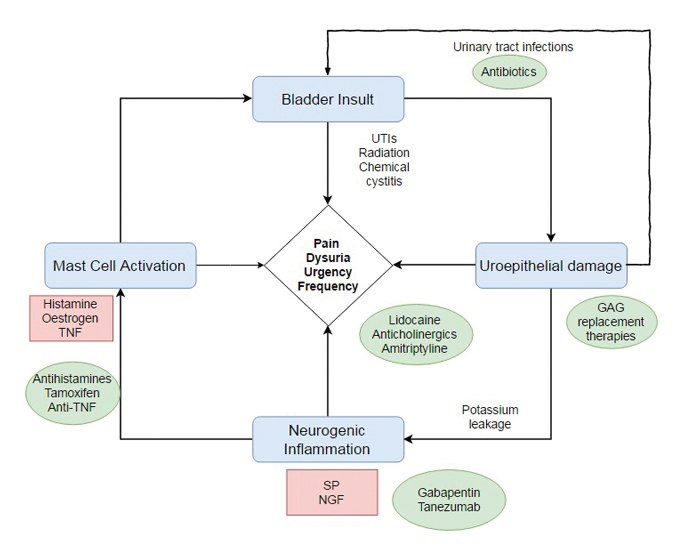
Figure 2: Putative mechanism of pathogenesis in recurrent cystitis (SP=substance P; NGF=nerve growth factor; TNF=tumour necrosis factor). Red boxes indicate potential substances that trigger symptoms. Green ovals indicate actual or potential therapeutic options.
Alternative intravesical therapies for recurrent cystitis
A better understanding of the pathogenesis of BPS has identified several putative processes downstream of uroepithelial GAG layer denudation which mediate and propagate bladder injury; these include neurogenic inflammation, afferent hyperexcitability and mast cell mediated pain. Therapeutic approaches in the future should reflect the multi-factorial aetiology of recurrent cystitis and considerable work is underway to identify novel pharmacological targets to complement existing treatment (Figure 2). For example, dimethyl sulphoxide (DMSO, also known as RIMSO-50) is an organosulphur compound used intravesically in BPS for its analgesic, anti-inflammatory and antioxidant properties. One RCT reported that DMSO provided statistically significant improvement in urinary symptoms as compared to placebo (53% improvement versus 18%) [21]. However, Cochrane review has failed to identify strong enough evidence to show significant benefit of this drug overall and it remains unlicensed in the UK despite it having an EAU Grade A recommendation for use in BPS.
Other examples of non-GAG therapeutic agents that have been assessed in studies include tanezumab, a NGF-neutralising monoclonal antibody, which has been shown to confer significant symptomatic relief over placebo in a double-blind phase II RCT of patients with BPS [22]. When given intravenously, tanezumab has a significant systemic side-effect profile and has been associated with headaches, paraesthesias, and case reports of bone necrosis; intravesical administration may minimise these side-effects by localising exposure to the agent. The use of intravesical anti-TNF biologics may also suppress local inflammatory processes in BPS without the risk of systemic immunosuppression. It is hoped that concomitant intravesical use of GAG replacement therapy and alternative disease-modifying therapies may produce a summative clinical benefit greater than if each were to be given alone.
Conclusion
Recurrent cystitis due to BPS or rUTI is often perceived as intractable as it is difficult to treat. The way forward should include RCTs and randomised comparative studies to select for the most efficacious intravesical treatments from the current catalogue. In addition, it is hoped that a better understanding of disease pathogenesis will allow for synergistic and targeted multi-factorial intravesical treatment to achieve an optimised therapeutic benefit for patients who suffer from recurrent cystitis.
References
1. Parsons CL. Prevention of urinary tract infection by the exogenous glycosaminoglycan sodium pentosanpolysulfate. J Urol 1982;127:167-9.
2. Nickel JC, Egerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int 2009;103:56-60. 3. Nickel JC, Egerdie RB, Steinhoff G, et al. A multicenter, randomized, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology 2010;76:804-9.
4. Engelhardt PF, Morakis N, Daha LK, et al. Long-term results of intravesical hyaluronan therapy in bladder pain syndrome/interstitial cystitis. Int Urogynecol J 2011;22:401-5.
5. Constantinides C, Manousakas T, Nikolopoulos P, et al. Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU Int 2004;93:1262-6.
6. Hanno P, Baranowski A, Fall M, et al. Painful bladder syndrome (including interstitial cystitis). In: Abrams PH, Wein AJ, Cardozo L (eds). Incontinence. 3rd edition. Paris, France; Health Publications Limited; 2005:1456-520.
7. Hwang P, Auclair B, Beechinor D, et al. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: A meta-analysis. Urology 1997;50:39-43.
8. Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo controlled study. The Journal of Urology 2015;193:857-62.
9. Simon M, McClanahan RH, Shah JF, et al. Metabolism of [3H]pentosan polysulfate sodium (PPS) in healthy human volunteers. Xenobiotica 2005;35:775–784.
10. Tasdemir S, Tasdemir C, Vardi N, et al. Intravesical hyaluronic acid and chondroitin sulfate alone and in combination for urinary tract infection: Assessment of protective effects in a rat model. International Journal of Urology 2012;19:1108-12.
11. Damiano R, Quarto G, Bava I, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol 2011;59:645–51.
12. Porru D, Leva F, Parmigiani A, et al. Impact of intravesical hyaluronic acid and chondroitin sulfate on bladder pain syndrome/interstitial cystitis. Int Urogynecol J 2012;23:1193-9.
13. Hauser PJ, Buethe DA, Califano J, et al. Restoration of the barrier function to acid-damaged bladder by intravesical chondroitin sulfate. J Urol 2009;182:2477-82.
14. Lai M-C, Kuo Y-C, Kuo H-C. Intravesical hyaluronic acid for interstitial cystitis/painful bladder syndrome: a comparative randomized assessment of different regimens. Int J Urol 2013;20:203–7.
15. Ciani O, Arendsen E, Romancik M, et al. Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case–control study. BMJ Open 2016;6:e009669.
16. De Vita D, Giordano S. Effectiveness of intravesical hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: a randomized study. Int Urogynecol J 2012;23:1707-13.
17. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int 2009;103:910-18.
18. Lv Y-S, Zhou H-L, Mao H-P, et al. Intravesical hyaluronic acid and alkalinized lidocaine for the treatment of severe painful bladder syndrome/interstitial cystitis. Int Urogynecol J 2012;23:1715–20.
19. Nomiya A, Naruse T, Niimi A, et al. On- and post-treatment symptom relief by repeated instillations of heparin and alkalized lidocaine in interstitial cystitis. Int J Urol 2013;20:1118–22.
20. Kuo HC. Urodynamic results of intravesical heparin therapy for women with frequency urgency syndrome and interstitial cystitis. J Formos Med Assoc 2001;100:309–14.
21. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol 1988; 140:36–39.
22. Evans RJ, Moldwin RM, Cossons N, et al. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol 2011;185:1716-21.
Further reading list
-
Evans RJ. Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol 2002;4:S16-S20.
-
Grover S, Srivastava A, Lee R, et al. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 2011;3:19-33.
TAKE HOME MESSAGE
-
Recurrent cystitits due to UTI or BPS is a difficult condition to treat, and having a full armamentarium of options, including intravesical therapy, is helpful.
-
GAG layer denudation is a putative mechanism of pathogenesis which has been implicated in rUTIs and BPS.
-
GAG replacement therapies have been shown to bind to damaged uroepithelium and restore bladder impermeability in mouse models. Several case series using intravesical GAG instillations to treat recurrent cystitis have shown positive outcomes.
-
Although there is a wealth of clinical experience with GAG replacement therapy accumulated over two decades, the evidence base for GAG layer replenishment remains weak and supported mainly by relatively small, uncontrolled studies. RCT evidence of efficacy over placebo is available only for HA-CS preparations. Firm conclusions will require larger well-powered trials as well as standardisation of treatment regimens and outcome measures.
-
Intravesical GAG instillations may be used as alternatives to long-term prophylactic antibiotics in recurrent bacterial cystitis to optimise outcomes and to prevent the development of antibiotic resistance.
-
Intravesical GAG replacement should be complemented with other pharmacological approaches to optimise therapeutic benefits in recurrent cystitis to reflect the multifactorial underlying aetiology.
Declaration of competing interests: None declared.