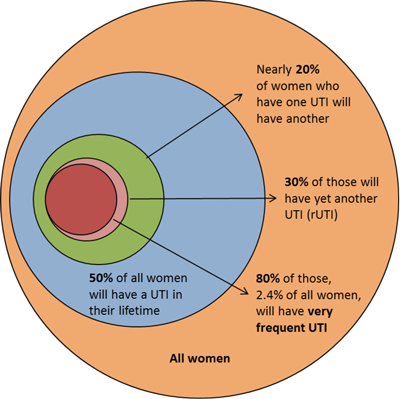
Acute uncomplicated infective cystitis is most commonly seen in healthy women with a frequency of around 0.5-0.7 episodes per woman per year [1]. Around 10% of women report having had an episode of urinary tract infection (UTI) each year and more than 50% of all women have at least one episode in their lifetime [2,3]. In contrast, men experience cystitis much less commonly [4].
Recurrent cystitis or recurrent urinary tract infection (rUTI) is generally regarded as having recurred if a second symptomatic infection follows clinical resolution of a previous one. Though there is no universally accepted definition, the occurrence of at least two episodes of acute uncomplicated cystitis within a six-month period, or at least three episodes in a 12-month period has been suggested [5]. rUTI is very common among young healthy women but rarely associated with anatomical or functional abnormalities of the urogenital tract. In the 1970s, a Danish study found that around half of women whose initial UTI resolved had recurrence in the first year [6]. Similar results were found in a Finnish study which demonstrated that women over 55 were particularly susceptible to recurrence [7].
Recurrent UTI occurs in one of two situations:
- Bacterial persistence
- Re-infection
Bacterial persistence is typically characterised by infection with the same organism recurring at very short intervals. In this case, identification and removal of reservoirs of infection will lead to resolution. In contrast, re-infections usually occur at more varied, longer intervals and may not necessarily be caused by the same organism. Re-infection, while more common in women than bacterial persistence, is a more complex situation where a single remediable abnormality is much less likely to be found. In men, rUTI is less common and most commonly associated with an underlying abnormality such as bladder outlet obstruction.
Figure 1: Urinary tract infection is very common in women
(adapted from Brumbaugh AR, Mobley HL [3]).
Bacterial persistence
Diagnosis
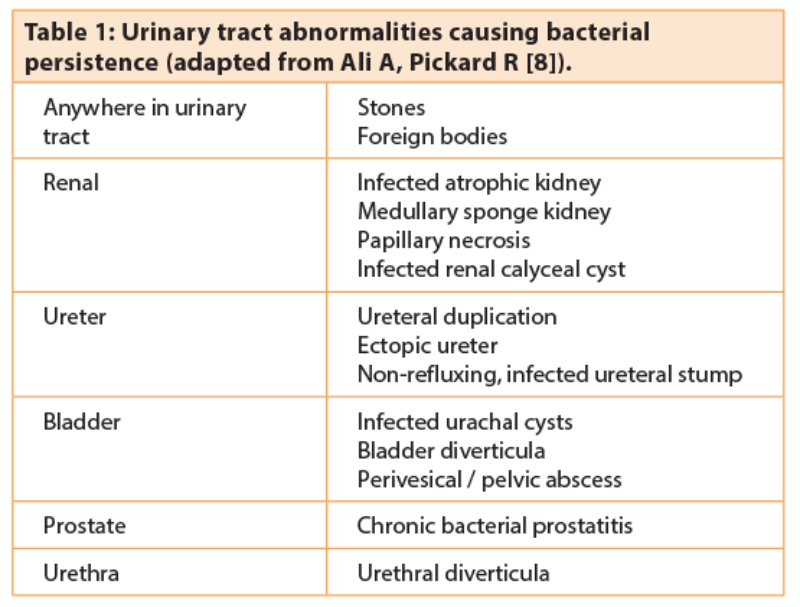
Once acute cystitis has resolved and there is no further microbiological evidence of bacteriuria, it is possible under certain circumstances for the organism to ‘hide’ within a part of the urinary tract which was exposed to less high concentrations of antimicrobial chemotherapy. The specific abnormalities that can result in this situation are outlined in Table 1 [8]. Urinary culture can be particularly useful in identifying bacterial persistence e.g. due to Proteus mirabilis, the commonest organism linked with the presence of infection stones. P. mirabilis can cause significant alkalinisation of the urine with precipitation of calcium, magnesium, ammonium, and phosphate salts and the subsequent formation of branched struvite (triple phosphate) renal stones. This has serious consequences as bacteria can persist inside struvite stones even when the urine shows no growth. Consequently, struvite infection stones are the major cause of bacterial persistence in women.
Many of the other abnormalities can be identified by a combination of imaging and endoscopic evaluation of the urinary tract. Although conventional intravenous urography may still be used, CT KUB (non-contrast) and CT urography (late phase contrast-enhanced) and cystoscopy provide the most sensitive investigation (particularly as struvite stones are often relatively radiolucent), retrograde urography and ureteroscopy are also useful in some situations.
Treatment
Where a reservoir for persistent infection is identified, the standard treatment is to remove the foreign body or correct the anatomical abnormality. Many of the abnormalities listed in Table 1 will require surgical intervention to facilitate removal and eradication of the source of bacterial persistence.
Where the reservoir of infection cannot be removed, long-term, low-dose antibiotic treatment may be the only option to supress bacterial growth and prevent symptoms. Agents typically recommended for this use are narrow-spectrum antibiotics such as nitrofurantoin and trimethoprim. Other drugs such as cefalexin, and the fluoroquinolones are avoided where possible due to higher risk of change in the natural flora.
Reinfections
Diagnosis
Recurrent infections occurring at longer intervals or by different bacteria are frequently indicative of reinfection and the diagnosis is therefore made clinically. It is most commonly secondary to ascending infection cause by coliforms especially Uropathogenic E. coli (UPEC), but less common causes such as fistulas (enterovesical or vesicovaginal) or other structural abnormalities are still possibilities to consider especially in patients with previous diverticulitis, surgery or radiotherapy. This is particularly true in men.
In younger women, sexually transmitted infections such as chlamydia trachomatis and neisseria gonorrhoeae can also cause rUTI type symptoms. Chlamydia is frequently asymptomatic in females, but both it and gonorrhoea can present with dysuria, discharge or pelvic inflammatory disease. Both organisms are best detected by PCR of urine and swab cultures. Chlamydia screening is recommended for all females under 25 years.
As with bacterial persistence, any urinary tract abnormalities which reduce the formation of urine or its flow through the urinary tract can increase the incidence of reinfection and limit the efficacy of antibiotic therapies. Ultrasound or radiographic imaging is useful to demonstrate the anatomy and basic functional status. Cystoscopy should also be performed where symptoms are suggestive of bladder obstruction, dysfunction, or fistula. Bladder cancer can present with persistent storage symptoms and incidental positive urine cultures mimicking rUTI.
Although many would consider cystoscopy necessary to rule out the occurrence of a complicated UTI, the diagnostic yield in many studies has been low leading some authors to suggest that it is not useful in these cases. In a study involving 74 cystoscopic evaluations Fowler et al. concluded that cystoscopy was not necessary in the investigation of rUTI [9]. This finding was supported in a study by Parsons et al. who investigated 244 women with rUTI and concluded that flexible cystoscopy did not reveal any relevant pathology [10]. Conversely, in a study by Lawrentschuk et al. involving 118 patients a “significant” abnormality was found in 8% of cases and cystoscopy was recommended especially in patients over 50 years [11]. The most recent guidelines published by the EAU however do not recommend the use of cystoscopy [12].
Treatment
As with most medical conditions, initial management should aim to correct reversible risk factors. This includes: glycaemic control in patients with diabetes, alternative contraception in women using spermicides or a diaphragm, review of management in patients with Foley catheters, possible oestrogen supplementation in women with post-menopausal vaginal atrophy and in elderly patients, hydration, faecal and urinary incontinence should be addressed.
General advice in terms of increased fluid intake, use of sanitary towels instead of tampons, post-coital micturition, and avoiding the use of soaps in the vaginal regions is also often given, though the associations with rUTI are weak [13,14,15].
When an infection occurs it should be treated with a full course of an appropriate antibiotic (as in the case of acute uncomplicated cystitis). After resolution of an acute episode, there are various treatment strategies to prevent recurrences which can be subdivided broadly into three categories:
- Non-antibiotic (and non-invasive) treatment
- Antibiotic based treatment
- Intravesical agents.
Non-antibiotic (and non-invasive) treatment
Cranberry products
Cranberry juice has been a popular preventive method for many years. It is believed to work by acidifying the urine and reducing bacterial adhesion. Cranberry juice contains proanthocyanidins which competitively inhibit the E. coli fimbrial subunit from binding to the uroepithelial cells and prevent expression of normal fimbriae [16]. Avorn et al. studied 153 women and showed that 300mL/day reduces bacteriuria and pyuria by 42% [17]. However, the actual cranberry content of juices and tablets is highly variable therefore effects are unpredictable. Furthermore, subsequent trials have not shown benefit and the latest Cochrane systematic review showed that overall cranberry products did not significantly reduce the occurrence of symptomatic UTI [18].
Oestrogen therapy
Postmenopausal women are recognised as a group more prone to frequent reinfections [19,20]. While in some cases, bladder or uterine prolapse contributes to residual urine after voiding, in others, the lack of oestrogen causes marked changes in the vaginal microflora and vaginal pH which leads to reduced lactobacilli and increased colonisation by E Coli [19]. Oestrogen replacement therapy is believed to restore the normal vaginal environment which allows re-colonisation with lactobacilli, reduces uropathogenic bacterial colonisation and hence incidence of UTIs [19]. However, the mechanism of action is not typical of a classical endocrine effect. Local oestrogen reduces UTI occurrence; systemic therapy does not [21]. The vaginal response is also rapid in onset but short-lived, lasting only for the duration of the therapy. The beneficial effects in rUTI are also seen in younger women using the oral contraceptive without evidence of oestrogen deficiency [22]. It is possible that oestrogen may also have actions on the innate immune defence mechanisms of the urinary tract [23]. Despite the unclear mechanism, the use of vaginal oestrogen in post-menopausal women is supported by Cochrane systematic review [21] and there may also be a role in pre-menopausal women.
Methenamine hippurate
As an alternative to the conventional antibiotics described below, evidence for the use of Methenamine hippurate (taken orally) also exists [24]. Methenamine is excreted by the kidney which decomposes at an acid pH to formaldehyde and ammonia, and the formaldehyde is bactericidal. Urinary acidity can be ensured by co-administering vitamin C (ascorbic acid) or ammonium chloride. Methenamine is particularly useful for long-term prophylaxis as bacteria do not develop resistance to formaldehyde. However, it should not be used in the presence of renal insufficiency and alkalinising agents should be avoided. In 2016, a randomised ‘non-inferiority’ clinical trial comparing this agent with conventional prophylactic antibiotics is being carried out in the UK with funding from the National Institute for Health Research (NIHR) [25].
Vaccines
The concept of vaccinating against UPEC is an attractive one but practically difficult to achieve given the primacy of the innate immune response (rather than the adaptive immunity) in the pathophysiology of UTI and the difficulty of therapeutic access to bladder epithelium to instigate a localised immune reaction. Nonetheless, the use of one oral vaccination approach has gained support from several studies and is included in the EAU guidelines [26]. This agent, known as Uro Vaxom® (OM-89), involves the oral administration (in capsule form) of immunologically active bacterial lysates of 18 E. coli strains. It has been shown to be more effective than placebo in several randomised control trials, the largest of which involved 453 women and showed a 34% reduction in UTI over 12 months compared to placebo [27]. The mechanism of action is not understood but is thought to innate immunity by increasing neutrophils and macrophage phagocytosis via up-regulation of dendritic cells. Another agent, Urovac®, is a vaginal vaccine containing 10 heat killed uropathogenic bacteria which are believed to induce IgG and IgA in the urogenital tract, thereby reducing potential colonisation of the vagina and bladder with uropathogens. Meta-analysis of three trials slightly reduced UTI recurrence (RR 0.81) but up to 27.8% of the women reported vaginal irritation [28]. Both ‘vaccine’ products have yet to be licensed in the UK.
Antibiotic based treatment
Low-dose continuous prophylaxis
Long-term prophylaxis is usually employed in the form of a single daily dose of antibiotic (typically taken at bedtime). The success of prophylaxis is dependent on the ability of an antimicrobial agent to eliminate pathogenic bacteria from the introital and bowel reservoirs without causing significant resistance. Evidence of efficacy exists for the following antimicrobials: trimethroprim, trimethroprim / sulphamethoxazole, nitrofurantoin and norfloxacin [29,30].
Long-term prophylaxis is typically continued for 6-12 months, although can be extended to several years. If symptomatic re-infection occurs during prophylactic treatment, then urine should be sent for culture and full therapeutic dosage of another antimicrobial should be used to treat the infection. Prophylaxis may then be resumed once the infection has resolved provided the culture results do not show the presence of resistance to the prophylactic agent. A Cochrane systematic review demonstrated that with prophylaxis [31] the relative risk of having one microbiological recurrence was 0.21 and for clinical recurrences was 0.15 with a number needed to treat of 1.85. Long-term prophylaxis is effective at preventing recurrences in 95% of patients whilst on prophylaxis but around 50% of patients will have an infection within three months once prophylaxis is discontinued [32].
Self-start therapy
Patient-initiated intermittent or self-start therapy is a useful alternative to long-term prophylaxis. It is recognised that 85-95% of women with previous UTI can self-diagnose successfully [33]. The key aim is to empower the patient to initiate a short course of empirical antibiotic treatment at the first onset of their symptoms or an ‘early warning sign’ which is consistent with development of previous episodes of cystitis [33,34]. The antimicrobial agent selected for patient-initiated therapy is essentially being used on an empirical basis therefore ideally it should have a broad spectrum of activity and achieve high urinary levels to minimise resistance whilst also having minimal impact on bowel flora. Nitrofurantoin and trimethoprim are common choices for patient-initiated therapy whereas tetracycline, ampicillin, and cephalexin in full doses are more likely to give rise to resistance [35].
In women where sexual intercourse has been identified as a ‘triggering event’ in the history, post-intercourse prophylaxis is a useful alternative form of self-start treatment [32]. A small dose of an antimicrobial such as nitrofurantoin, trimethoprim, or a fluoroquinolone immediately after voiding post-intercourse can effectively reduce reinfection [36] with efficacy comparable to daily prophylaxis and fewer side-effects [37].
Intravesical agents
It is well known that significant numbers of bacteria are able to invade the bladder epithelium and persist in the bladder after an acute UTI [38]. It is postulated that these quiescent intracellular bacteria may contribute to UTI recurrence and that damage to the glycosaminoglycan layer (GAG) may be involved in both UTI recurrence and interstitial cystitis. Disruption of the GAG layer may expose epithelial cells to infectious urine components and increase bacterial adherence [39] thus playing a role in the aetiology of rUTI. As is the case with the treatment of interstitial cystitis, it is proposed that mucopolysaccharides such as hyaluronic acid (HA) and chondroitin sulphate (CS), which are components of the extracellular matrix and constitute an important component of bladder GAG layer, may be used intravesically to enhance the protective function of the urothelium. Intravesical agents currently available for use are: Cystistat® (HA only), Hyacyst® (HA only), iAluril® (HA & CS) – all three agents are delivered via catheter. A recent systematic review of four studies by De Vita et al. demonstrated decreased UTI rate per patient-year (mean difference 3.41), significantly longer mean UTI recurrence time (mean difference 187.35 days), and pelvic pain and urgency / frequency (PUF) total score [40]. However, it was noted that the analysis was limited by the small number of patients introducing possible bias. It was concluded that “further studies are needed to validate this promising treatment modality.”
Other strategies
In patients where the post-micturition residual urine is judged to be significant and due to a narrowing of the urethra, a single dilation of the urethra to improve bladder emptying is reasonable. There is little evidence, however, that repeated urethral dilation is indicated in the routine management of most women. There is also currently no evidence that factors such as frequency of voiding, timing of voiding, wiping patterns, use of hot baths, or type of undergarments, play any significant role in rUTI. There is therefore no rationale for giving women specific instructions regarding these factors.
Conclusion
To conclude, while rUTI remains a common and troublesome problem affecting quality of life in women particularly, a number of management options are now available. There is a growing evidence base for these treatments with many having undergone systematic reviews and meta-analyses. In common with other urological conditions, a stepwise approach can be adopted, beginning with conservative and non-antibiotic based treatments, moving onto antibiotics with the most invasive intravesical measures reserved for those with the worst symptoms. Using such an approach should help restore quality of life in the majority of sufferers.
References
1. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New England Journal of Medicine 1996;335:468-74.
2. Foxman B, Barlow R, D'Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000;10(8):509-15.
3. Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines 2012;11(6):663-76.
4. Krieger JN, Ross SO, Simonsen JM. Urinary tract infections in healthy university men. J Urol 1993;149(5):1046-8.
5. Hooton TM, Stamm WE. Recurrent Urinary Tract Infection in Women. In: Rose BD (Ed). UpToDate, Waltham, MA, USA; UpToDate; 2006.
6. Mabeck CE. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad Med J 1972;48:69-75.
7. Ikaheimo R, Sutonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996;22:91-9.
8. Ali A, Pickard R. Infection of the lower urinary tract. In Turner N, Goldsmith D, Winearls C, et al. (Eds). Oxford Textbook of Clinical Nephrology Oxford, UK; Oxford University Press; 2015:1495-505.
9. Fowler JE Jr, Pulaski ET. Excretory urography, cystography, and cystoscopy in the evaluation of women with urinary-tract infection: a prospective study. N Engl J Med 1981;304(8):462-5.
10. Parsons SD, Cornish NC, Martin B, Evans SD. Investigation of uncomplicated recurrent urinary tract infections in women. Journal of Clinical Urology 2016 [online].
11. Lawrentschuk N, Ooi J, Pang A, et al. Cystoscopy in women with recurrent urinary tract infection. Int J Urol 2006;13(4):350.
12. Grabe M, Bartoletti R, Bjerklund Johansen TE, et al. Guidelines on Urological Infections. EAU 2015.
13. Remis RS, Gurwith MJ, Gurwith D, et al. Risk factors for urinary tract infection. American Journal of Epidemiology 1987;126:685-94.
14. Strom BL, Collins M, West SL, et al. Sexual activity, contraceptive use, and other risk factors for symptomatic and symptomatic bacteriuria. Annals of Internal Medicine 1987;107:816-23.
15. Foxman B, Chi JW. Health behaviour and urinary tract infections in college-aged women. Journal of Clinical Epidemiology 1990;43:329-37.
16. Patel N, Daniels I. Botanical perspectives on health of cystitis and cranberries. Journal of Royal Society of Health 2000;120:52-3.
17. Avorn J, Monane M, Gurwitz J, et al. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA 1994;271:751-4.
18. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2012;10:CD001321.
19. Hooton TM, Stam WE. Management of acute uncomplicated urinary tract infection in adults. Med Clin North Am 1991;75(2):339-57.
20. Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med 1993;329:753-6.
21. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev 2008;2:CD005131
22. Pinggera GM, Feuchtner G, Frauscher F, et al. Effects of local estrogen therapy on recurrent urinary tract infections in young females under oral contraceptives. Eur Urol 2005;47:243-9.
23. Lüthje P, Hirschberg AL, Brauner A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas 2014;77(1):32-6.
24. Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev 2012;10:CD003265.
25. http://www.nets.nihr.ac.uk/projects/hta/138821
26. Grabe M, Bartoletti R, Bjerklund Johansen TE, et al. Guidelines on Urological Infections. Arnhem, Netherlands; European Association of Urology; 2015.
27. Bauer HW, Alloussi S, Egger G, et al. Multicenter UTI Study Group. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol 2005;47(4):542-8.
28. Beerepoot MA, Geerlings SE, van Haarst EP, et al. Non-antibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol 2013;190(6):1981-9.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: Diagnosis and treatment. Infect Dis Clin North Am 1987;1:793-806.
30. Nicolle LE, Harding GK, Thompson M, et al. Prospective, randomized, placebo-controlled trial of norfloxacin for the prophylaxis of recurrent urinary tract infection in women. Antimicrob Agents Chemother 1989;33(7):1032-5.
31. Albert X, Huertas I, Pereiró II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004;3:CD001209.
32. Nicolle LE. Urinary tract infection: traditional pharmacologic therapies. American Journal of Medicine 2002;113(1A):35S-44S.
33. Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Annals of Internal Medicine 2001;135:9-16.
34. Schaeffer AJ, Stuppy BA. Efficacy and safety of self-start therapy in women with recurrent urinary tract infections. Journal of Urology 1999;161:207-11.
35. Wong ES, McKevitt M, Running K, et al. Management of recurrent urinary tract infections with patient-administered single-dose therapy. Ann Intern Med 1985;102:302-7.
36. Pfau A, Sacks T, Engelstein D. Recurrent urinary tract infections in premenopausal women: Prophylaxis based on an understanding of the pathogenesis. J Urol 1983;129:1153-7.
37. Melekos MD, Asback HW, Gerharz E, et al. Post-intercourse versus daily Ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. Journal of Urology 1997;157:935-9.
38. Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1999;282:1494-7.
39. Parsons CL. Epithelial coating techniques in the treatment of interstitial cystitis. Urology 1997;49:100-4.
40. De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for recurrent bacterial cystitis in adult women: a meta-analysis. Int Urogynecol J 2013;24(4):545-52.
Declaration of competing interests: None declared.