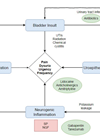
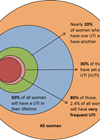
Urinary tract infections (UTIs) occur when bacteria colonise and proliferate in the urinary tract. These are characterised by specific clinical symptoms (dysuria, suprapubic tenderness, urgency and urinary frequency) which commonly occur alongside the finding of bacteriuria. UTIs are common – 150 million people worldwide are diagnosed with one every year [1]. Closer to home, they account for 15% of antibiotic prescribing in the community and make up 1-3% of all GP consultations in the UK [2].
As with most bacterial infections, UTIs are treated with antibiotics; local guidelines vary, but generally in the UK, nitrofurantoin or trimethoprim are viewed as first-line treatments, with aminoglycosides (like gentamicin) second-line options. The majority of UTIs are uncomplicated and can be managed in the community, but more severe or complicated cases can require hospital admission for intravenous antibiotics.
If not treated sufficiently, UTIs can spread causing acute or chronic pyelonephritis, cystitis, urethritis, epididymitis, prostatitis, abscess formation or urosepsis. In young patients, recurrent infections can cause renal scarring which can lead to hypertension and chronic renal insufficiency [3]. In specific situations, sequelae of untreated UTI can have long-term impact on the individual’s future health, and may even be fatal. Early recognition and appropriate antibiotic therapy is important in preventing these outcomes.
UTIs are diagnosed by a combination of clinical assessment, urine dipstick analysis and/or midstream urine microscopy, culture and sensitivities (MC&S). Diagnosing UTIs on clinical symptoms alone can lead to misdiagnosis and inappropriate use of antibiotics for presumed infection, such as when patients complain of recurrent UTIs when on reflection, they have other pathologies common confused with infection, such as overactive bladder syndrome or the painful bladder syndrome/interstitial cystitis.
Furthermore, bacteria may be present in the urine in the absence of symptoms and an estimated 10% of men and 20% of women aged over 65 are estimated to have asymptomatic bacteriuria [4]. Whilst asymptomatic bacteriuria may require to be treated in certain situations, such as when discovered incidentally during preoperative assessment for planned surgery, the vast majority of cases do not require antibiotic therapy.
Guidelines
In the UK, the National Institute for Health & Care Excellence (NICE) recommend that urine dipstick results are not used in isolation to diagnose UTIs in adults over 65, nor used at all in the diagnosis of those with urinary catheters. These patients require a full clinical assessment to identify the features of a UTI [4] and ideally, a urine sample sent for MC&S prior to starting antibiotic therapy. The accuracy of urinalysis can vary greatly (especially in older patients, non-pregnant women and those with catheters) so treatment should ideally only be given when clinical symptoms are also present. In a similar vein, NICE advise against the use of prophylactic antibiotics in patients with catheters unless they have a history of severe or recurrent infections [4].
Recent guidelines from NICE have recommended that healthcare staff, where possible, tailor antibiotics according to patient characteristics, symptom severity, complication risk and previous antibiotic use with the aim of reducing antibiotic resistance [5].
The guidance states that many cases of cystitis in non-pregnant women are self-limiting so ‘back-up prescriptions’ can be given to be used in the case of worsening symptoms or if there is no improvement in 48 hours. The decision between back-up and immediate prescribing should consider the severity of symptoms, the risk of developing complications and previous culture results.
The recommendation is that samples should be sent for culture and bacterial sensitivities gained prior to antibiotic prescription (in cases where symptoms are mild and risk of complications low). This allows the creation of a regimen tailored to each patient, but obviously would require someone responsible for chasing up the MCS urine results, which can add significant workload pressures to an already stretched system. Such ‘back up’ therapy, needless to say, is not appropriate in severe or complicated infections [5].
For pregnant women or in men, when an UTI is suspected, the advice is to send a midstream urine for culture and start an antibiotic immediately (taking into account any previous cultures taken). Once the microbiology sensitivities are back the choice of antibiotic can be revised to more effectively target the specific invading organism [5].
Antibiotic resistance
Rising rates of resistance to antibiotics have been recorded since the 1990s [6]. It is estimated that 10 million lives per year will be at risk from antibiotic-resistant infections by 2050 [7]. Escherichia coli is the most commonly isolated urinary organism. NICE surveillance data has found that over a third of E. coli UTIs are resistant to commonly used antibiotics [5]. This increasing incidence of resistance poses a problem for management of UTIs, in particular strains that produce extended-spectrum beta-lactamase (ESBL) and those resistant to fluoroquinines such as ciprofloxacin.
In 2013, the resistance rates for community-acquired E. coli UTIs across Canada were found to be 16%, a recorded rise of 5.2% since 2002 [8]. Such rises are also reflected in the UK, where studies on community-acquired UTIs in children found a 43% prevalence of E. coli resistant to at least one antibiotic (most often amoxicillin, co-amoxiclav or trimethoprim) and a 17% prevalence of the multi-drug resistant organisms (resistant to more than three antimicrobials) [7].
Resistant organisms are seen more often in complicated UTIs and in those who have previously been treated with antibiotics.
Interestingly, studies have shown antibiotics may promote treatment resistance by causing alterations in the gut flora. Promotion of a development of a gut reservoir of resistant bacteria enables UTIs to occur through autoinfection [8]. Other risk factors for infections with resistant organisms include the presence of a long-term catheter, hospitalisation within the last year, congenital urinary malformations or complex reconstructions, previous UTIs or regular antibiotic prophylaxis [3].
Antibiotic-resistant UTIs are associated with a higher morbidity and mortality than their antibiotic-sensitive counterparts [9]. Studies show that in general practice, patients with highly resistant UTIs remain symptomatic for longer than those with infections caused by sensitive organisms (to first-line antibiotics) [10,11]. Treating UTIs inappropriately or by unnecessary use of prophylactic antibiotics leads to selection of resistant organisms which exacerbates the problem.
Furthermore, patients with resistant UTIs are more likely to re-consult at the GP / Emergency Department [10,11]. As the number of resistant infections increases, the workload associated with treating UTIs is set to increase, including an increased hospital admission rate [6].
It seems that resistant strains can be found globally with higher rates of resistant E. coli being found in those who have recently travelled to certain locations in the world, as specific resistance patterns are dependent upon geography and regional antibiotic usage [8].
With increasing resistance rates and multi-drug resistant bacteria now present in the community as well as in acute settings it is becoming more difficult to know which antibiotics are appropriate to prescribe. The World Health Organization states that to find the best treatment for individuals it is crucial to have local data on antimicrobial incidence and resistance [12]. This can be used to generate good recommendations for rational antibiotic use and local treatment guidelines.
Antibiotic-free alternatives
With the rapidly rising rates of antibiotic resistance there is a pressing need to find alternative therapies to use as prophylaxis against UTIs. An open access review on current and new therapies in treating UTIs has recently been published [13]. Below we explore some potential antibiotic-free preventative options.
D-Mannose
D-Mannose is a naturally occurring sugar found within human metabolism. It has been found both in vitro and in vivo to prevent UTIs. Initially used in cats, dogs and horses, D-Mannose prevents adherence to the bladder urothelial cells by inhibiting the fimbriae on the surface of E Coli. This may occur in the bladder, but also it may be occurring in the gut.
In a large randomised controlled trial (RCT), it was found in 308 women that 2g of D-Mannose diluted in 200mls of water taken daily for six months produced a risk reduction of 0.24 in getting UTIs when compared to placebo [14]. D-Mannose is readily available over the counter.
Hiprex
Methenamine hippurate (Hiprex) prevents UTIs when excreted in the kidneys. Methamine salts undergo hydrolysis to form formaldehyde, which harbours bactericidal properties. Furthermore, the Hippuric acid formed acidifies the urine creating a bactericidal effect as well as promoting the hydrolysis of methamine salts.
The current dosage recommendation is 1g twice a day, increasing to three times a day if a catheter is present. A Cochrane Review involving 2032 patients found a relative risk of UTI on treatment of 0.24 in previously symptomatic patients [15].
Oestrogen creams
An atrophic vaginal micro-environment occurs in menopausal women, where the lack of oestrogen stimulation results in a loss of the natural acidic defence created by lactobacillus as well as loss of vaginal architecture. Vaginal oestrogen (applied either as a pessary ring for 12 weeks or 0.5mg oestriol nightly for two weeks, then twice a week for 6-12 months) has been shown to restore the pre-menopausal vaginal state and reinstate the natural defences. A Cochrane Review in 2008 of 3345 women showed a risk reduction between 0.25–0.64 when compared against placebo. Interestingly no significant benefit was found in oral systemic oestrogen therapy, which of course is linked with carcinoma and thromboembolic risks and are not indicated for prevention of recurrent UTIs [16].
Vaginal CO2 fractional laser
CO2 ablation lasers (such as FemTouch™ and MonaLisa®) create fractional micro trauma to the vaginal mucosa. The subsequent “trauma” has been found to induce the vaginal thick squamous stratified epithelium to restore itself. This restoration can be found in vaginal biopsies taken after treatment.
Essentially the pre-menopausal vaginal condition is restored. The Cleveland clinic is currently running a multicentre trial, comparing vaginal laser and vaginal oestrogen creams in a single blinded prospective manner [17].
The first prospective study of a CO2 vaginal laser in 12 postmenopausal women with recurrent UTIs have found three courses at monthly intervals resulted in 75% of patients remaining UTI free in the 12-month follow-up period. This was associated with improvement in the Vaginal Health Index Score in line with restoration of the vagina [18]. However, the use of this technology in UTIs is still very much in its infancy and larger, well-powered studies are needed before this can be offered in routine practice.
Instillation therapy
It has been theorised that bacterial adherence to the epithelial cells in the bladder is prevented by an overlying intact layer of glycosaminoglycan (GAG). It has therefore thought that the presence of a damaged GAG layer increases the risk of UTIs; this is based on studies in animal models. Instillations to restore the GAG layer are commonly used for the treatment of interstitial / radiation cystitis and overactive bladder and are currently commercially available. These include Cystistat®, a sodium hyaluronate solution, and iAluRil®, composed of a mixture of hyaluronic acid and chondroitin sulphate. Both agents are instilled into the bladder via an ‘in-out’ catheter.
A large retrospective European study of 276 women with recurrent urinary tract infections found that UTI recurrences reduced when intravesical treatments were used (odds ratio 0.77 vs. placebo) [19]. A retrospective study involving 151 patients with recurrent UTIs suggests a superior rate of infection after intravesical chondroitin sulphate in comparison to antibiotic prophylaxis [20]. Further double-blind RCTs are required to confirm the role of GAG layer replacement therapy.
Although all these products require urethral catheterisation, selected patients can self-catheterise and self-administer the treatment provided they adhere to the recommended instillation regime – this enables reduction of cost and patient empowerment in their own treatment.
Interestingly, iAluRil has recently developed a 'catheter free' syringe to allow instillation of the treatment without the need for an invasive catheter. This method is similar to injecting Instillagel® into the urethra; however; more clinical outcome data from this new device are still awaited.
Immunomodulation
Current research is exploring means of stimulating the body’s own immune system to fight UTIs.
Uro-vaxom®
Originally called OM-89 in literature, Uro-vaxom is composed of extracts from eight pathogenic E. coli strains in a tablet. This is taken once a day for three months, stimulating an immune response from the body to prevent further infections. A 2005 double blinded RCT in 453 patients found decreased UTI rates when compared with a placebo [21]. However, a recent RCT of 451 patients did not show any difference in UTI reduction compared to placebo; although the overall rates of UTI in this new study was low which may have skewed the data [22].
Uromune®
Previous research has shown stimulation of the mucosal associated lymphoid tissue (MALT) induces a similar response at a distant MALT site [23]. Two sites which have been found to be linked are the sublingual mucosa and the genitourinary tract. Stimulation therefore of the MALT at the sublingual mucosa induces a distant-immunological response as well in the genitourinary tract. Uromune works by stimulating the sublingual mucosa.
Uromune is a new immunomodulation therapy administered via a sublingual pineapple flavoured spray. It is composed of inactivated whole bacteria comprising the four most common uropathogenic organisms – Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris and Enterococcus Faecalis.
Previous large retrospective studies in Spain, comparing Uromune with antibiotics prophylaxis in 319 and 699 women, showed risk reduction of 77% after 15 months and 90.3% after 12 months follow-up, respectively [24,25]. A recent UK prospective study in 77 women with recurrent UTIs found a daily sublingual spray for three months led to 78% of patients reporting no subsequent UTIs in the 12-month follow-up period [26]. Currently Uromune is only available as an unlicensed product on a named patient basis. Presently there are no randomised placebo-controlled trials, although one phase III study is currently underway looking uncomplicated recurrent UTIs in females and is expected to report final results in 2019 / 2020.
Conclusion
UTIs are common and are encountered by virtually all doctors. Diagnosis should be made from both clinical features and urine dipstick analysis, with the use of urine cultures to direct antibiotic therapy. A tailored approach is ideal, especially because often symptoms of cystitis are self-limiting.
Rising antibiotic resistances rates worldwide, compounded and aided by overuse of antibiotics, is putting greater pressure on health systems and is creating a new global health crisis. Fortunately, there are several alternatives to antibiotics which are currently being explored.
References
1. Gonzales CM, Schaeffer AJ. Treatment of urinary tract infection; what’s new, what’s old, what works. World Journal of Urology 1999;17(6):372-82.
2. Mazzulli T. Resistance trends in urinary tract pathogens and impact on management. Journal of Urology 2002;168(4 Pt 2):1720-2.
3. Lutter SA, Currie ML, Mitz LB, Greenbaum LA. Antibiotic resistance patterns in children hospitalised for urinary tract infections. Archives of Pediatric and Adolescent Medicine 2005;159(10):924-8.
4. NICE. Urinary tract infections in adults. Quality Standard [QS90]. 2015.
www.nice.org.uk/guidance/qs90
Accessed 10 June 2018.
5. Test urine before prescribing antibiotics for most UTIs, says NICE. BMJ 2018;361:k2076.
6. Hooton TM, Besser R, Foxman B, et al. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clinical Infectious Diseases 2004;39(1):75-80.
7. Nicolle LE. Antimicrobial resistance in community-acquired Escherichia coli isolated from urinary infection: Good news or bad? Canadian Journal of Infectious Diseases and Medical Microbiology 2013;24(3):123-4.
8. Bryce A. Comparison of risk factors for, and prevalence of, antibiotic resistance in contaminating and pathogenic urinary Escherichia coli in children in primary care: prospective cohort study. Journal of Antimicrobial Chemotherapy 2018;73(5):1359-67.
9. Lin E, Bhusal Y, Horwitz D, et al. Overtreatment of enterococcal bacteriuria. Archives of Internal Medicine 2012;172(1):33-8.
10. Butler CC, Hillier S, Roberts Z, et al. Antibiotic-resistant infections in primary care are symptomatic for longer and increase workload: outcomes for patients with E.coli UTIs. British Journal of General Practice 2006;56(530):686‑92.
11. McNulty CAM, Richards J, Livermore DM, et al. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. Journal of Antimicrobial Chemotherapy 2006;58(5):1000-8.
12. Antibiotic Resistance. WHO Online Factsheet. 2018.
www.who.int/en/news-room/
fact-sheets/detail/antibiotic-resistance
Accessed 10 June 2018.
13. Yang B, Foley S, Toozs-Hobson P. Urinary tract infections: current and new preventative options. SM Journal of Clinical Medicine 2016;2(2):1018.
14. Kranjcec B, Papes D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World Journal of Urology 2014;32(1):79-84.
15. Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Systematic Review 2012;10:CD003265.
16. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Obstetrics & Gynecology 2008;112(3):689-90.
17. Comparison of Vaginal Laser Therapy to Vaginal Estrogen Therapy (VeLVET). The Cleveland Clinic. 2017.
18. Yang B, Foley C, Foley S. Using the fractional thermo-ablative laser FemTouch™ as a novel treatment in women with recurrent Urinary Tract Infections: The first experience in the United Kingdom. European Association of Urology 2018.
19. Ciani O, Arendsen E, Romancik M, Biase, et al. Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case-control study. BMJ Open 2016;6(3):e009669.
20. Rahnama’i M, Rozenberg B, Röschmann K, Arendsen H. Retrospective evaluation of efficacy and safety of intravesical 0.2 % chondroitin sulphate solution in urinary tract infections in comparison to treatment of UTI with long-term low-dose antibiotics. European Urogynaecological Association 2016.
21. Bauer HW, Alloussi S, Egger G, et al. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. European Urology 2005;47(4):542-8.
22. Wagenlehner FM, Ballarini S, Pilatz A, et al. A randomized, double-blind, parallel-group, multicenter clinical study of escherichia coli-lyophilized lysate for the prophylaxis of recurrent uncomplicated urinary tract infections. Urologia Internationalis 2015;95(2):167-76.
23. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Natural Medicine 2005;11(4 Suppl):S45-53.
24. Lorenzo-Gomez MF, Padilla-Fernandez B, Garcia-Criado FJ, et al. Evaluation of a therapeutic vaccine for the prevention of recurrent urinary tract infections versus prophylactic treatment with antibiotics. International Urogynecology Journal 2013;24(1):127-34.
25. Lorenzo-Gomez MF, Padilla-Fernandez B, Garcia-Cenador MB, et al. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front Cell Infect Microbiol 2015;5:50.
26. Yang B, Foley S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune. BJU International 2018;121(2):289-92.
Declaration of competing interests:
Bob Yang and Steve Foley have received reimbursement of travel expenses from Syner Med to attend conferences.