Definitions
Infertility is the inability of a sexually active, non-contracepting couple to achieve spontaneous pregnancy in one year [1]. About 15% of couples do not achieve pregnancy within one year and seek medical treatment for infertility. Semen parameters are standardised by the World Health Organization (WHO) and defined according to standardised values discussed in the investigation section below.
Azoospermia is defined as the absence of sperm in the ejaculate and is identified in 10% to 15% of infertile males [2].
Oligospermia is defined as a sperm density of less than 15 million/ml. Unlike the situation with azoospermia, causes of oligospermia can be quite vast and the aetiology is often idiopathic.
Asthenospermia is decreased sperm motility (<32% motile spermatozoa [3]).
Teratospermia is abnormal forms of sperm (<4% normal forms [3]).
Sperm abnormalities of oligospermia, asthenospermia and teratospermia usually occur together; this is called oligo-astheno-teratozoospermia (OAT) syndrome.
In 50% of infertile couples, a male associated factor is found together with abnormal semen parameters [3].
Physiology
The hypothalamic-pituitary-gonadal (HPG) axis is responsible for reproductive tract formation and development, maturation of fertility potential at puberty, and the maintenance of sexual function in the adult. The HPG axis is illustrated in Figure 1.
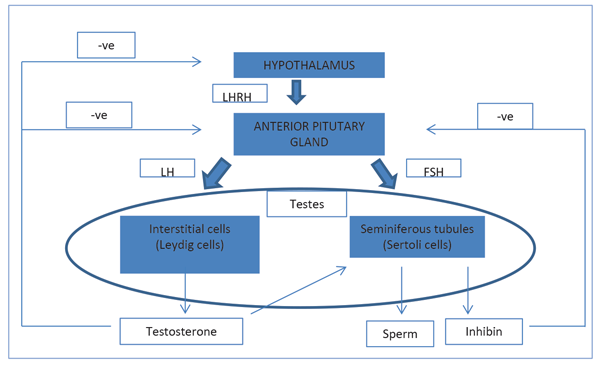
Figure 1. The hypothalamic-pituitary-gonadal (HPG) axis.
The hypothalamus secretes gonadotrophin-releasing hormone (GnRH). This causes the release of follicle stimulating hormone (FSH) and luteinising hormone (LH) from the anterior pituitary gland, which act on the testis. LH acts on Leydig cells to produce testosterone. FSH stimulates the seminiferous tubules to secrete inhibin and produce sperm.
Testosterone is secreted by the interstitial Leydig cells. It promotes the development of the male reproductive system and secondary sexual characteristics. Sixty percent of testosterone is bound to sex hormone binding globulin (SHBG), 38% bound to albumin and 2% is free. Testosterone is converted into a more potent androgen, dihydrotestosterone, by 5-alpha reductase at the target tissues.
Sertoli cells line the seminiferous tubules, which surround developing germ cells (spermatogonia) and provide nutrients and stimulating factors as well as secreting androgen-binding factor and inhibin. Primordial germ cells divide to form primary spermatocytes. These undergo two meiotic divisions to form spermatids and a further differentiation into spermatozoa. This process takes 72 days. The non-motile spermatozoa leave the seminiferous tubules and pass to the epididymis for storage and maturation.
Aetiology
Pre-testicular causes:
- Endocrinopathy / hormonal, hypogonadotropic hypogonadism.
- Systemic disease e.g. renal failure; liver cirrhosis; cystic fibrosis (CF).
- Environmental factors e.g. hot baths.
- Drugs, alcohol, smoking and cannabis.
- Genetic abnormalities e.g.
- Klinefelter’s syndrome (47 XXY) characterised by small firm testes, gynaecomastia and high serum gonadotrophins.
- Deletions in the azoospermic factor (AZF) gene on the Y chromosome. Microdeletions of region AZFa has been associated with Sertoli cell only syndrome; AZFb with maturation arrest and AZFc with azoospermia or severe oligozoospermia.
Testicular causes:
- Varicoceles are found in 40% of infertile men [4]. Varicoceles may be associated with failure of testicular growth, hypogonadism or symptoms of pain and discomfort.
- Idiopathic 30-40% [1].
- Undescended testes (cryptorchidism).
- Functional sperm disorder e.g. antisperm antibodies; Karatagener’s syndrome.
- Testicular injury e.g. testicular torsion.
- Infection e.g. post pubertal mumps orchitis.
- Radiation.
- Cancer.
- Post surgery e.g. hernia repair.
Post-testicular (obstructive causes):
- Male genital tract obstruction e.g. obstruction / absence of vas deferens (may be associated with CF). CF is an autosomal recessive disorder that affects multiple organs. Most men with azoospermia with congenital bilateral absence of vas deferens (CBAVD) have genetic mutation in cystic fibrosis transmembrane conductance regulator (CFTR) on chromosome 7p.
- Erectile or ejaculatory problems e.g. retrograde ejaculation.
- Infection e.g. prostatitis.
Furthermore, the level of obstruction in obstructive azoospermia (OA) varies:
- Intratesticular obstruction 15%.
- Epididymal obstruction 30-67%.
- Vas deferens obstruction: most common cause of acquired obstruction following vasectomy.
- Ejaculatory duct obstruction: 1-3%
Assessing male infertility
History
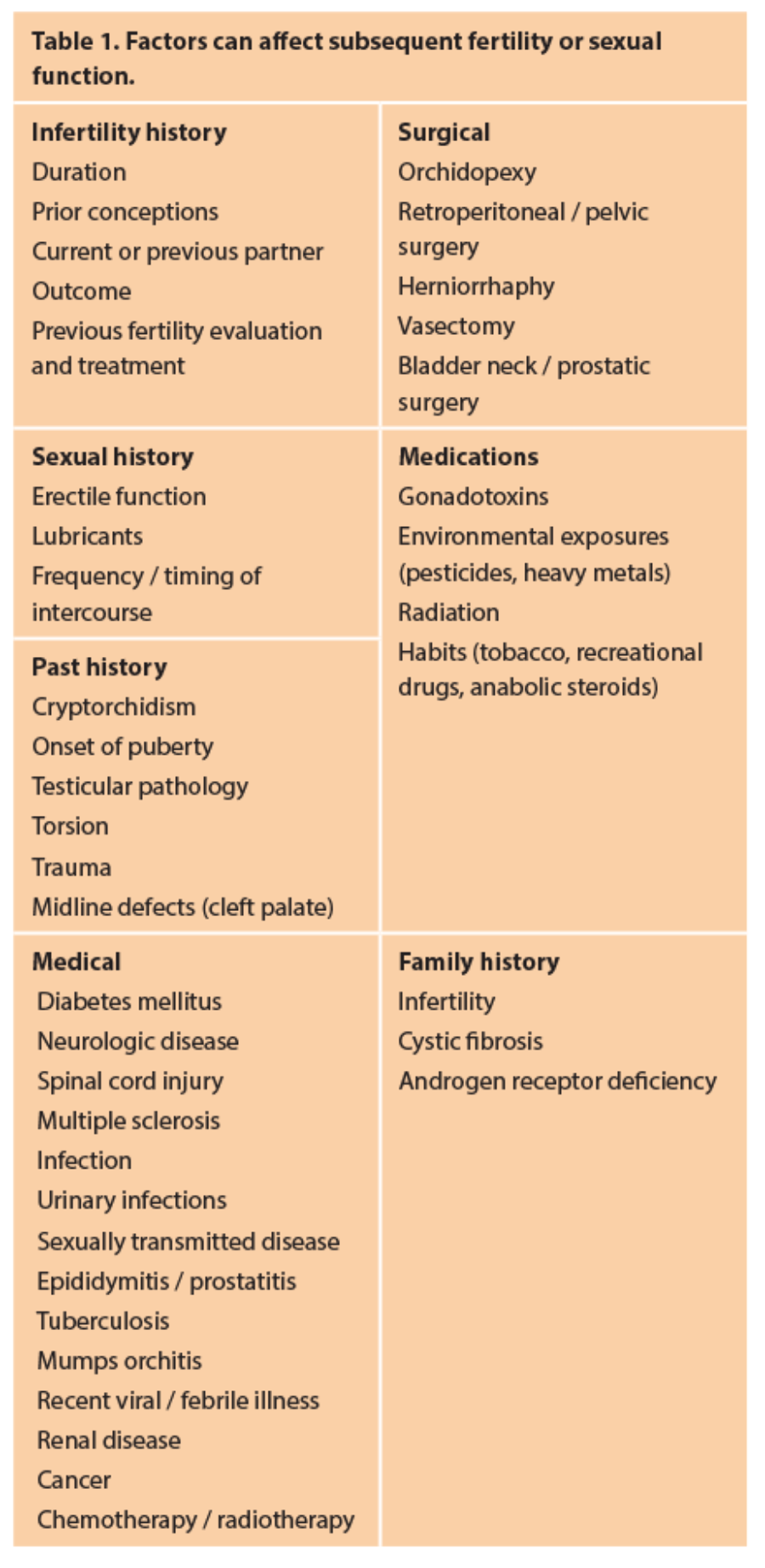
A thorough history is key in successful diagnosis of male infertility. Many specific factors can affect subsequent fertility or sexual function (Table 1).
Examination
General examination It is important to perform a comprehensive general examination with particular attention to:
- Evidence of secondary sexual development.
- Gynaecomastia.
- Signs of hypogonadism.
- Decreased body hair, absence of temporal pattern balding areas – document secondary sexual characteristics.
- Low androgen levels at the time of puberty will lead to disproportionately long extremities due to delayed closure of the epiphyseal plates.
- Examination of thyroid gland may exclude nodules suggesting hyperfunction or hypofunction, which can affect fertility.
- Hepatomegaly on abdominal examination raises suspicion for hepatic dysfunction, which may induce altered sex steroid metabolism.
Genital examination
- Penis: curvature, Peyronie’s plaque, phimosis, hypospadias.
- Testes: assessment of testicular consistency, tenderness and volume (using an orchidometer or by sonographic measurement; normally >20ml) and to exclude the presence of testicular masses.
- Epididymis: tenderness and fullness.
- Spermatic cord: presence or absence of vas deference, varicocele.
Investigations
There are a number of available tools to further evaluate the infertile male, ranging from the basic semen analysis to testicular biopsy, as well as imaging studies. Basic investigations: Appropriate laboratory testing of semen plays a key role in evaluation of men presenting with infertility.
- Semen analysis: specimens should be collected over a period of a few weeks after two to five days of sexual abstinence. The specimen should be delivered within one hour to the laboratory. The normal values for semen parameters are shown in Table 2.
- Hormonal assessment: these include LH, FSH and testosterone. Increased prolactin levels can be associated with sexual dysfunction and may indicate pituitary disease. Men with testicular deficiency have high levels of FSH and LH, and sometimes low levels of testosterone secondary to hypergonadotropic hypogonadism. Generally, the levels of FSH correlate with the number of spermatogonia: when spermatogonia are absent or markedly diminished, FSH values are usually elevated; when the number of spermatogonia is normal, but maturation arrest exists at the spermatocyte or spermatid level, FSH values can be within the normal range. Table 3 tabulates the different endocrine profiles in infertile men.
Special investigations:
- Genetic evaluation for Y microdeletion, karyotype and CF transmembrane conductance regulator (CFTR) gene.
- Testicular biopsy: In selected cases, testicular biopsy may be indicated to exclude spermatogenic failure (non-obstructive causes). Testicular biopsy should be performed at the time of testicular sperm extraction and be part of intracytoplasmic sperm injection (ICSI) treatment in patients with clinical evidence of non obstructive azoospermia (NOA). Microdissection testicular sperm extraction (TESE) is the technique of choice. Spermatogenesis may be focal, which means that in about 50% of men with NOA, spermatozoa can be found and used for ICSI.
- Post-orgasmic urine analysis to confirm retrograde ejaculation in men with low ejaculatory volume.
- Sperm function tests, which are not commonly performed, include:
1. Post coital test
2. Sperm penetration test
3. Sperm-cervical mucus test
Radiological investigations:
- Ultrasound scan of the scrotum: assess testicular abnormality (e.g. mass) and the presence of varicocele, information on which should include venous diameter and evidence of venous reflux.
- Transrectal ultrasound if seminal volume is low to exclude obstructive causes e.g. Müllerian duct cyst.
- Vasography: This is to diagnose and evaluate level of obstruction if obstructive azoospermia is suspected to confirm distal patency prior to reconstruction e.g. vaso-epididymostomy.
Management
Treatments vary according to the underlying cause and the degree of the impairment of the male fertility. Pre-testicular conditions and idiopathic cases may respond to medical therapy:
- Gonadotropin-releasing hormone agonists: These agents are effective for treatment of hypogonadotropic hypogonadism.
- Anti-oestrogens: Anti-oestrogens remain the most commonly employed medical therapy for idiopathic male infertility e.g. clomiphene citrate and tamoxifen citrate. A meta-analysis reported some improvement in sperm quality and spontaneous pregnancy rates [5]. However, these are unlicensed.
- Oral antioxidants: Vitamins e.g. vitamin E (daily 400mg), vitamin C (1gm twice daily) and co-enzyme Q10 (CoQ10 200mg once daily) [6].
Testicular conditions (and idiopathic infertility that do not respond to medical treatment) can be treated with assisted reproduction techniques (ART).
Azoospermia
Sperm extraction from the epididymis is performed by:
- Percutaneous epididymal sperm aspiration (PESA): This involves aspirating fluid from the epididymis through a percutaneous approach.
- Microsurgical epididymal sperm aspiration (MESA): This is an open surgical sperm retrieval procedure from the epididymal tubules under the microscope.
The above two techniques are useful approaches when there is CBAVD or vasectomy reversal is not an option.
Sperm extraction from the testes is performed by:
- Testicular exploration and sperm extraction (TESE / microsurgical TESE or Aspiration TESA).
- A TESE involves extracting testicular tissue from multiple areas through an open approach or under the microscope in mTESE. A systematic review showed that sperm retrieval ranged from 16.7 to 45% in the conventional TESE vs. 42.9 to 63% in the microTESE group [7].
- The retrieved sperm is stored by cryopreservation or used at the same time as ICSI.
Post testicular conditions can be managed with either surgery or IVF-ICSI.
Varicocoeles can be repaired by embolisation; open, laparoscopic or microsurgical operations. A meta-analysis has shown that microsurgical varicocoelectomy technique has a higher pregnancy rate and is associated with lower recurrence rates and hydrocele formation [8].
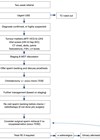
Varicocoelectomy has been the subject of much debate. Surgical varicocoelectomy of clinical varicoceles has been shown to result in a significant improvement of semen parameters [9-11], although subclinical varicocoeles remain a matter for debate [12]. It has been postulated that there is also a significant risk of over treatment of adolescents with varicocoeles [13]. Figure 2 illustrates the algorithm for evaluation and treatment of azoospermia.
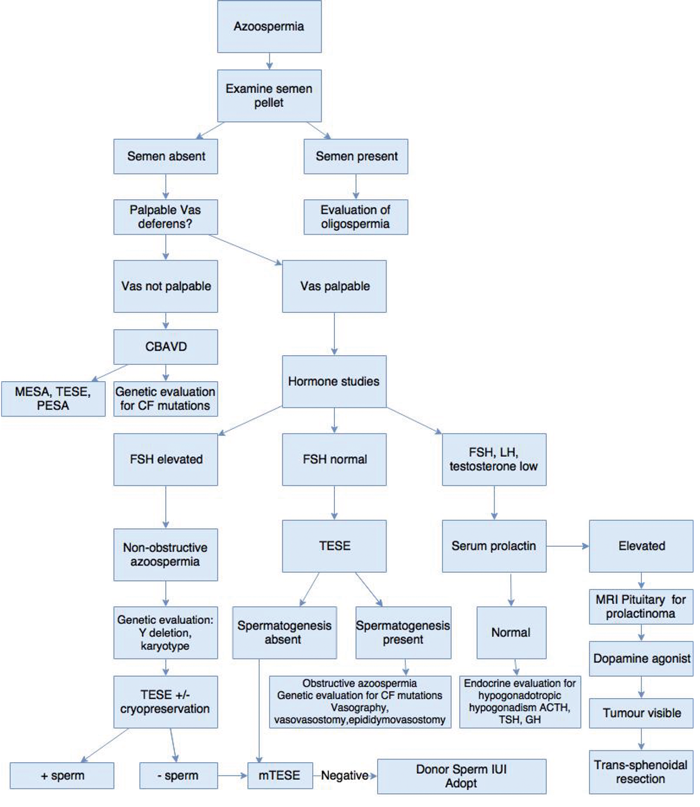
Figure 2. Algorithm for evaluation and treatment of azoospermia.
The management of obstructive azoospermia depends on the level of obstruction:
Intratesticular obstruction: Only TESE provides a means of obtaining sperm.
Proximal vas deferens obstruction: Proximal vas deferens obstruction after vasectomy requires microsurgical vasectomy reversal. The length of time elapsed since vasectomy is the major factor determining success rates from surgery. A patency rate of 97% and a pregnancy rate of 76% was achieved following reversal in an obstructive interval of three years, whereas these rates where lower (patency rate 71%, pregnancy rate 30%) if this interval was 15 years or more [14]. The absence of spermatozoa in the intraoperative vas deferens fluid suggests the presence of a more proximal obstruction where a vaso-epididymostomy (anastomosis of the vas to the epididymis) or a microsurgical tubulovasostomy will be required. The presence of spermatozoa is confirmed by examining the fluid from the testis end of the vas under the microscope intraoperatively.
Distal vas deferens obstruction: TESE / MESA or proximal vas deferens sperm aspiration can be used for cryopreservation for future ICSI as it is often difficult to correct large vas deferens defects e.g. following hernia repair.
Ejaculatory duct obstruction: Transurethral resection of the ejaculatory ducts (TURED) can be used in men with ejaculatory duct obstruction e.g. intra-prostatic midline cyst. Complications following TURED include retrograde ejaculation due to bladder neck injury and urine reflux into the ejaculatory ducts, seminal vesicles, and vasa. The alternatives to TURED are MESA, TESE, ultrasonically guided aspiration of the seminal vesicle and direct midline cyst aspiration; although using these latter techniques, cysts tend to recur.
In cases of obstruction due to a midline intraprostatic cyst, incision or deroofing of the cyst is required. Intra-operative transrectal ultrasound (TRUS) makes this procedure safer.
Installation of methylene blue dye into the seminal vesicle can aid confirming patency of the ducts. The limited success rate of surgical treatment of ejaculatory duct obstruction in terms of spontaneous pregnancies should be weighed against sperm aspiration and ICSI.
References
1. World Health Organization. WHO Manual for the Standardised Investigation and Diagnosis of the Infertile Couple. Cambridge, UK: Cambridge University Press; 2000.
2. Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. The Journal of Urology 1989;142(1):62-5.
3. Jungwirth A, Giwercman A, Tournaye H, et al.European Association of Urology Working Group on Male Infertility. European Association of Urology guidelines on Male Infertility: the 2012 update. European Urology 2012;62(2):324-32.
4. Wein A, Kavoussi L, Novick A, et al. Campbell-Walsh Urology, 10th Edition Philadelphia, USA: Elsevier Saunders; 2012.
5. Chua ME, Escusa KG, Luna S, et al. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013;1(5):749-57.
6. Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int 2014;2014:426951.
7. Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: a systematic review. Andrology 2014;2(1):20-4.
8. Cayan S, Shavakhabov S, Kadioglu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl 2009;30(1):33-40.
9. Agarwal A, Deepinder F, Cocuzza M, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology 2007;70(3):532-8.
10. Baazeem A, Belzile E, Ciampi A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. European Urology 2011;60(4):796-808.
11. Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl 2016;18(2):246-53.
12. Yamamoto M, Hibi H, Hirata Y, et al. Effect of varicocelectomy on sperm parameters and pregnancy rate in patients with subclinical varicocele: a randomized prospective controlled study. The Journal of Urology 1996;155(5):1636-8.
13. Ding H, Tian J, Du W, et al. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU International 2012;110(10):1536-42.
14. Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. The Journal of Urology 1991;145(3):505-11.
Take home message
-
History, examination and the semen are the key aspects of diagnosing male factor infertility. These, in addition to hormonal profile, would likely determine the diagnosis of oligospermia or azoospermia (non-obstructed vs. obstructed).
-
Available algorithms provide a pivotal tool for diagnosis and management.
-
In treating male factor infertility, the female partner’s age has an influence on the choice of intervention.
-
Eligibility for NHS funding should be considered and discussed with the patient.
-
In obstructive azoospermia, the choice of intervention depends on the level of obstruction.
-
Surgical sperm retrieval techniques vary in success rates and the use of microscopy is a valuable adjunct.
Declaration of competing interests: None declared.