Subfertility or infertility is a major problem affecting men diagnosed with testicular cancer (TC) either due to the disease itself [1], or as a result of management [2]. TC is the most prevalent cancer affecting men of reproductive age [3]. Incidence rates increase drastically after 15 years of age, and peak between 30-34 years of age before sharply declining [3].
Testicular cancers arise from germ cells into either seminomas (50%) or non-seminoma tumours (e.g. teratomas) (40%); mixed tumours also occur representing 10% of cases [4].
Incidence rates of testicular cancer and infertility
The lifetime risk of developing testicular cancer is 1 in 215, which accounts for less than 1% [5] of cancer diagnoses in the UK. In 2015, there were 2228 cases of testicular cancer [3]. Spermatogenesis was not present in 21-38% of men with TC [2,6-8]. As a result, there are increased rates of azoospermia and oligospermia (over 50% [9]), meaning that infertility rates within this patient population rates are between 10-35% [1].
The relationship between tumours and fertility
Spermatogenesis has been found to occur in all types of germ cell tumours [2]. However, spermatogenesis is most likely to occur away from the tumour site within the testis (50.0% away from the tumour versus 1.4% within the tumour) [2]. Statistically significant inverse relationships between tumour size and spermatogenesis have been shown in multiple studies [6-8]. Interestingly, TC even has an effect on contralateral testis spermatogenesis in up to 24% of men [10].
The effects of testicular cancer treatment on fertility
Postoperatively, patients may require either chemotherapy or radiotherapy both of which affect fertility. It is estimated that patients having a 30% decrease in fertility post chemotherapy compared to the time of diagnosis [2]. However, it is difficult to directly quantify the effect of chemotherapy on fertility as men who undergo chemotherapy have more advanced disease, which may also impact their fertility. A recent review found that <850mg doses of cisplatin led to higher conception rates than >850mg [11], but this is difficult to quantify definitely given the heterogeneity between chemotherapy regimens. Similarly, whilst is it proven that radiotherapy affects fertility, the exact impact is unknown as studies show contradicting results [12]. Table 1 highlights conception rates.
Table 1: Observed conception rates >10 years after treatment for TC (adapted from Moody et al. [11])
Treatment: Average combination
Observed conception rates: 66-71%
Treatment: Radical orchiectomy
Observed conception rates: 80%
Treatment: Radical orchiectomy + RPLND
Observed conception rates: 59-78%
Treatment: Cisplatin <=850mg
Observed conception rates: 73%
Treatment: Cisplatin >=850mg
Observed conception rates: 42-56%
Treatment: Average cisplatin-based therapy
Observed conception rates: 85%
Treatment: Radiotherapy
Observed conception rates: 64-66%
Treatment: Radiotherapy + cisplatin therapy
Observed conception rates: 56%
Treatment: Radiotherapy +/- RPLND + radiotherapy
Observed conception rates: 68%
Treatment: Radical orchiectomy +/- RPLND + cisplatin
Observed conception rates: 50%
Depending upon tumour types and staging, some men require retroperitoneal lymph node dissection (RPLND) in conjunction with a radical orchiectomy. This can lead to retrograde ejaculation (RE) unless a nerve sparing approach is followed as the retroperitoneal sympathetic nerve complex can be damaged (>90% infertility rates are reported when bilateral non-nerve sparing RPLND are performed [12]). Following nerve-sparing approaches, normal ejaculation has been reported in 77-99% of patients [12].
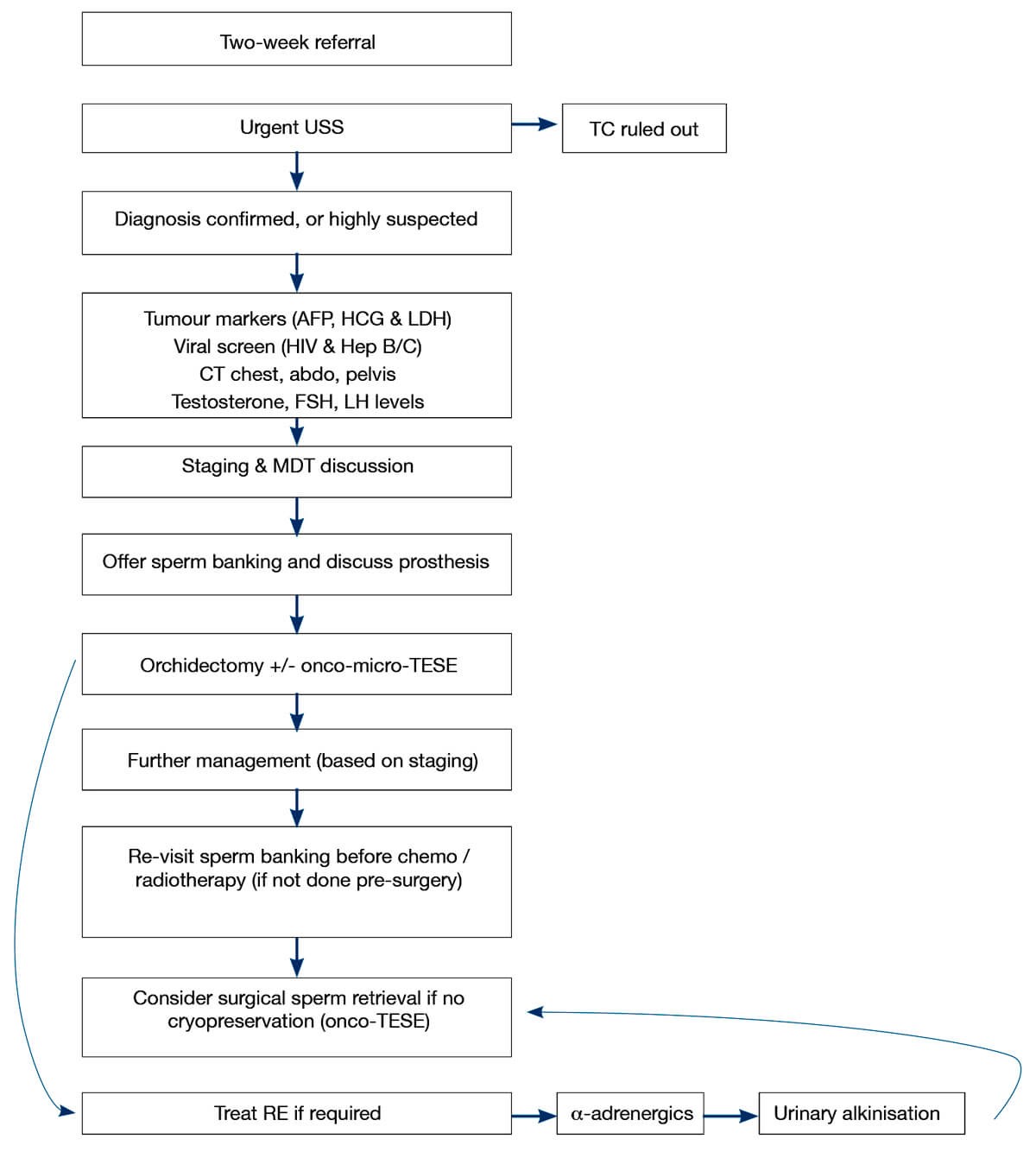
Figure 1: Summary of the management of pathway options for fertility treatment in TC.
Management options of infertility
Given that the majority of TC patients are of child bearing age, and 51-71% wish to father children [11], it is paramount to attempt to maintain fertility (Figure 1). Cryopreservation is the most effective method to do this. Ideally, it should be done preoperatively as sperm quality has been found to be decreased post orchidectomy [13]. It is important to note that whilst cryopreservation is not as effective postoperatively, it is still better than no cryopreservation at all. Regardless, cryopreservation is greatly under-utilised with only 24-30% TC patients banking their sperm [11].
If cryopreservation is not done initially, an alternative to sperm banking is surgical sperm extraction. Multiple studies have reported success rates of 66-85.7% using testicular sperm extraction (TESE) in postoperative azoospermic patients (even those who had undergone chemotherapy) [11]. Another option is onco-micro-TESE which entails performing TESE at the time of the orchidectomy, in areas of the testis which are not affected by the tumour. In these instances, spermatogenesis has been found in the affected testis 62-80% of the time and allows for maximum sperm preservation, without the need for further surgical management [11]. If required, it is possible to perform a normal micro-TESE if no sperm is found in the affected testis.
If men have undergone RPLND and are suffering from retrograde ejaculation it should be treated initially pharmacologically with α-adrenergics (e.g. pseudoephedrine). If this is not successful, urinary alkalinisation enables sperm to be collected from the bladder. Should this not be suitable, patients may be suitable for surgical sperm retrieval [11].
Success rates of fertility management
Cryopreservation preoperatively or intraoperatively allows men to undergo assisted conception when they wish to conceive. Men with suitable semen can choose to undergo intra-uterine insemination whereas those with fewer high quality sperm can undergo intracytoplasmic sperm injection (ICSI). In men who underwent cryopreservation preoperatively, success rates of 31-82% have been reported [12].
Whilst not specific to TC, for men who choose to have cryopreservation and then have chemotherapy, success rates for artificial insemination and ICSI are reported to be 11.1% and 30.5% live birth rates respectively [14]. Importantly, these rates did not vary compared to fresh ejaculated sperm rates. Similarly, in men with TC who have undergone chemotherapy and then have had a micro-TESE combined with ICSI, live birth rates of 29-42% have been reported [12].
It is important to note that patients are still able to conceive naturally after undergoing TC treatment varying from 50-85% (10 years post treatment) depending upon the treatment undergone.
Cryopreservation issues
The poor uptake of cryopreservation can be attributed to many reasons. Firstly, far too few men are referred to the service, with only 42-50% of oncologists discussing cryopreservation [11]. It is especially concerning that those with worst prognoses are the least likely to be referred [11]. Ultimately, it is a man’s choice if he wishes to cryopreserve and 50% of men do not wish to [15]. Other reasons that men decline cryopreservation include wanting to start chemotherapy (31%), not being offered (18%) and cost (9%) [15]. In the UK, the cost for this is borne by the NHS.
Psychological issues related to TC
Like most cancers, testicular cancer is known to cause psychological issues. As can be expected, men who are unable to father children are affected by this, with 51.9% men reporting moderate-severe distress [16]. Furthermore, men who achieve paternity have a significantly improved quality of life compared to those who do not achieve paternity [17]. A related issue is that men can develop erectile dysfunction post TC treatment [18], which further affects their chances of conceiving.
Summary
Testicular cancer is the most prevalent cancer in men of reproductive ages and has a major impact of fertility. However, proper preoperative counselling provides men with the opportunity of cryopreservation in the NHS and identifies azoospermic / oligospermic men, who could benefit from an onco-micro-TESE. Even if these approaches are not followed, surgical sperm retrieval is still an option. All of these options allow men to father children successfully, which has been shown to improve their quality of life.
TAKE HOME MESSAGE
-
Testicular cancer is the most prevalent cancer in fertile males and can significantly affect fertility.
-
Men need to be properly counselled regarding these issues and their options to maintain fertility.
-
Preoperatively, patients’ baseline fertility must be assessed to see which fertility treatment is most appropriate.
-
There are many options to maintain fertility; cryopreservation is preferable but if this is not possible, there are many alternatives that can be pursued, even postoperatively.
-
Men should be reviewed and counselled regularly regarding fertility during all stages of treatment – it is never too late.
References
1. Spermon JR, Kiemeney LA, Meuleman EJ, et al. Fertility in men with testicular germ cell tumors. Fertil Steril 2003;79(Suppl 3):1543-9.
2. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer – predictors of spermatogenesis. BJU International 2018;122(2):236-42.
3. Cancer Research UK. Testicular cancer incidence statistics. 2019.
www.cancerresearchuk.org/
health-professional/cancer-statistics/
statistics-by-cancer-type/
testicular-cancer/
incidence#heading-One
[accessed 12 April 2019].
4. Dearnaley D, Huddart R, Horwich A. Regular review: Managing testicular cancer. BMJ (Clinical research ed) 2001;322(7302):1583-8.
5. Cancer Research UK. Testicular Cancer Risk Cancer Research UK. 2019.
www.cancerresearchuk.org/
health-professional/cancer-statistics/
statistics-by-cancer-type/
testicular-cancer/
risk-factors#heading-Zero
[accessed 20 May 2019].
6. Choy JT, Wiser HJ, Bell SW, et al. Predictors of spermatogenesis in orchiectomy specimens. Urology 2013;81(2):288-92.
7. Delouya G, Baazeem A, Boman JM, et al. Identification of spermatozoa in archived testicular cancer specimens: implications for bench side sperm retrieval at orchiectomy. Urology 2010;75(6):1436-40.
8. Shoshany O, Shtabholtz Y, Schreter E, et al. Predictors of spermatogenesis in radical orchiectomy specimen and potential implications for patients with testicular cancer. Fertil Steril 2016;106(1):70-4.
9. Howell SJ, Shalet SM. Effect of cancer therapy on pituitary–testicular axis 1. International Journal of Andrology 2002;25(5):269-76.
10. Berthelsen JG. Testicular cancer and fertility. Int J Androl 1987;10(1):371-80.
11. Moody JA, Ahmed K, Yap T, et al. Fertility management in testicular cancer: the need to establish a standardized and evidence-based patient-centric pathway. BJU International 2019;123(1):160-72.
12. Hamano I, Hatakeyama S, Ohyama C. Fertility preservation of patients with testicular cancer. Reproductive Medicine and Biology 2017;16(3):240-51.
13. Rives N, Perdrix A, Hennebicq S, et al. The semen quality of 1158 men with testicular cancer at the time of cryopreservation: results of the French National CECOS Network. J Androl 2012;33(6):1394-401.
14. Schmidt KL, Larsen E, Bangsboll S, et al. Assisted reproduction in male cancer survivors: fertility treatment and outcome in 67 couples. Hum Reprod 2004;19(12):2806-10.
15. Sonnenburg DW, Brames MJ, Case-Eads S, Einhorn LH. Utilization of sperm banking and barriers to its use in testicular cancer patients. Supportive Care in Cancer 2015;23(9):2763-8.
16. Fegg MJ, Gerl A, Vollmer TC, et al. Subjective quality of life and sexual functioning after germ-cell tumour therapy. British Journal of Cancer 2003;89(12):2202-6.
17. Stoehr B, Schachtner L, Pichler R, et al. Influence of achieved paternity on quality of life in testicular cancer survivors. BJU Int 2013;111(4 Pt B):E207-12.
18. Tal R, Stember DS, Logmanieh N, et al. Erectile dysfunction in men treated for testicular cancer. BJU Int 2014;113(6):907-10.
Declaration of competing interests: None declared.