The rising incidence of childhood cancer coupled with increasing survivorship means there is a growing population of childhood cancer survivors with unmet health needs. In the UK around 1 in 500 children and young people are survivors of childhood cancer. So great is this increase in survivorship, that a growing body of work is now focused on promoting post-cancer treatment quality of life.
Cancer treatment protocols include chemotherapy, radiotherapy, and / or surgery; as well as targeting the cancer, these modalities can have unwanted and off-target effects. Importantly, the impact of these effects may not manifest until many years after treatment. This is particularly so in childhood cancers, for which the full effects of treatment may not be realised until adulthood.
One often overlooked impact of cancer treatment is its detrimental impact on fertility; however, there is clear evidence that patients treated for cancer are less likely to go on to become pregnant or father a pregnancy. For adult men, fertility preservation is often a critical concern when undergoing gonadotoxic treatments which may diminish their ability to produce sperm. Care pathways are well established to cryopreserve semen for future fertility care in men due to receive gonadotoxic treatment, with demonstratable success using these sperm for in assisted reproductive technology (ART). However, pre-pubertal boys are unable to produce sperm and for these children facing similarly gonadotoxic treatment, options for fertility preservation are limited.
Moving beyond the barriers – potential techniques to restore fertility
There is increasing evidence for the use of cryopreserved gonadal tissue, rather than gametes, for fertility preservation and restoration; in female patients, over 200 live births worldwide have been reported from use of ovarian tissue which has been cryopreserved prior to cancer treatment and then re-transplanted after the patient has been cured of their disease [1]. Testicular tissue cryopreservation is now being offered to prepubertal boys in a number of centres globally, with increasing numbers of patients undergoing the procedure and evidence to suggest there is no detrimental impact of the biopsy on ongoing testis development [2]. However, storage of testicular tissue cannot yet be considered an established method of fertility preservation given that the use of this tissue to restore human fertility has not been demonstrated.
Pre-pubertal males who are offered tissue storage as a means of fertility preservation are subject to differences in practice from centre to centre, including variations in eligibility, storage methods and criteria for future use. Furthermore, funding for such novel (and what many would consider experimental) treatments is highly variable between countries. Given the nature of childhood cancer and the primary concern being survival from their disease, it is understandable that clinicians and parents may be concerned about pursuing fertility preservation options if they are perceived to risk delaying treatment. This is further complicated by the age (peak at 0-4 years) of the childhood cancer patient population and the amount of time before any detrimental effects to fertility may be apparent or fatherhood is considered. When making decisions about treatment, fertility preservation will be just one of multiple competing priorities and it may be challenging for families to see it as an immediate concern.
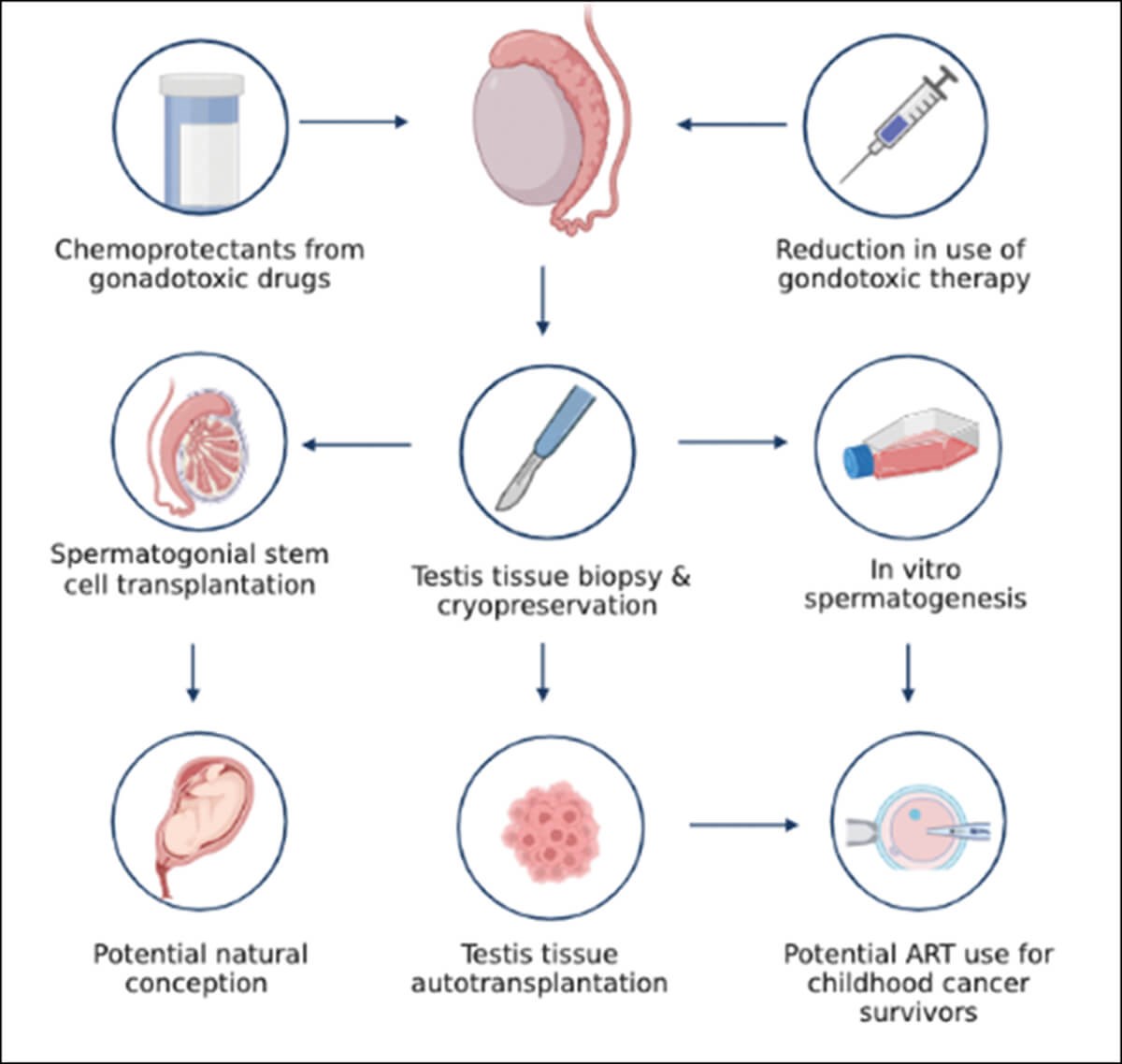
Figure 1: Fertility preservation options.
It is estimated that one in three pre-pubertal boys with cancer will experience fertility issues as an adult. But it is challenging to predict which individual patients will go on to experience these effects. Chemotherapeutics frequently used in childhood cancer such as platinum-based chemotherapy and alkylating agents are known to be gonadotoxic; they can result in loss of spermatogonial stem cells (SSC), which are required for continuous sperm production in adulthood. This loss of SSCs may be due to direct damage or as a result of injury to the cells in the testis that support sperm development, the so-called ‘SSC niche’. Furthermore, risk to fertility can be affected by duration of therapy, disease site, type of cancer, and other treatment modalities employed (such as cranial or pelvic / gonadal irradiation; surgery; and other potentially gonadotoxic drugs). Patients may initially receive a relatively low risk treatment regimen but have a disease relapse which necessitates use of more intensive and gonadaotoxic treatments. This makes identifying those at risk of infertility challenging and introduces barriers to targeted fertility preservation.

Figure 2: The Edinburgh team storing testicular tissue from a patient for research.
Recent advances using in vitro and animal models highlight advances made in this area and offer patients with testicular tissue cryopreserved hope in terms of future fertility. Of these exciting developments, the birth of Grady, a rhesus macaque born using sperm derived from cryopreserved prepubertal testis tissues, which had been autotransplanted back into the donor monkey, demonstrates how fertility may be achieved in humans [3]. These proposed techniques anticipate that in vivo factors will support graft survival and differentiation of SSC into spermatozoa, but there are several issues to be overcome. Firstly, autologous grafting is currently limited to animal models, and while these results are promising, success in animal models does not necessarily translate into success with human tissues. In fact, prior to optimising these techniques in humans there are numerous, clinical, ethical, and regulatory barriers to be overcome, including the risk of reintroducing residual malignant disease. The use and development of these techniques will be limited by the availability of tissue, requiring those wishing to undergo autologous grafting to have had testis tissues cryopreserved prior to receiving sterilising treatment.
Given the limitations of whole tissue transplantation, other novel approaches such as in vitro spermatogenesis have been explored as an alternative and may be an avenue to future fertility success. In vitro spermatogenesis again utilises cryopreserved testis tissues to allow the SSCs to differentiate into spermatozoa to be used in assisted reproductive technologies. Despite the experimental nature of this technique, animal studies have yielded promising and exciting results, with researchers being able to achieve germ cell differentiation from cryopreserved testis tissues into cells exhibiting characteristics of mature spermatozoa and capable of producing offspring after ART [4]. Although these results are promising, this technique is not without barriers to be overcome prior to use in humans, including optimising culture conditions for human tissue to successfully generate functional sperm and the potential for detrimental impacts on offspring both in the short- and long-term.

Figure 3: Members of the ORCHID-NET consortium at the
European Testis Workshop, Caux, Switzerland, 2023.
The final technique we discuss is SSC transplantation and perhaps the only technique which may offer restoration of natural fertility, avoiding the need for ART. Given the loss of SSCs due to treatment, transplantation of cryopreserved SSCs back into the seminiferous tubules has been performed in mice. Although the numbers of SSCs present within the mouse prepubertal testis, as a proportion of the total cell number within the testis is lower than in humans; the limited number of SSCs available to transplant and challenges in accurately identifying these cells represents a significant issue when employing this technique. One way to overcome this is to expand the number of SSC available to transplant by first culturing these cells in vitro, prior to transplanting them back into the testis; however, as with in vitro spermatogenesis, this is limited by tissue availability and the need to establish robust techniques to repopulate the testis with colony forming SSCs.
The bigger picture – harmonising research efforts for clinical impact
Of the many novel techniques under development to preserve and restore fertility, barriers to achieving success in this field are not solely that of an experimental nature. Globally, the storage of cryopreserved tissue is variable. Countries that do offer fertility preservation often have variation in patient eligibility criteria and funding for this, and consensus is required in relation to biopsy techniques, tissue storage conditions, length of storage, criteria for future use and assessment of tissue function or sperm quality should these tissues be used in ART. Variation in practice is not uncommon in reproductive medicine or indeed in male infertility. A recent systematic review of outcome reporting in the 100 largest randomised controlled trials in the last 10 years demonstrated significant heterogeneity in clinical outcome selection, reporting and measurement, with only four of these trials reporting live birth as an outcome, despite 16 of these trials reporting clinical pregnancy rate [5]. Inconsistencies in trial design, clinical outcome reporting, definitions of outcomes and how these are measured can hinder evidence synthesis across studies and limit its implementation into clinical practice. This has led to the development of core outcome sets, minimum data sets to be used and reported on in future clinical trial design to reduce research waste and lead to the identification of clinically impactful interventions.
While clinical trial design and outcome reporting may be of use in the future, the field of paediatric fertility preservation is in its infancy. Which begs the question, what does the need for core outcome set development in adult fertility research have to do with paediatric fertility preservation? Given our understanding as to the importance of methodological consistency and transparent reporting to derive reproduceable interventions with meaningful clinical impact in adult fertility research, new initiatives to harmonise research in paediatric onco-fertility preservation are now being developed, aiming to minimise the impacts of variation in study design and reporting. To this end, ‘ORCHID-NET’ (https://www.orchid-net.com/) has been established as a global collaboration of clinicians, scientists and methodologists, with expertise in pre-pubertal male reproductive biology and an interest in developing fertility preservation strategies for young males facing gonadotoxic treatments. Although these initiatives may be new, they signal a growing understanding and anticipation that novel techniques used in animal models may soon be translated into human studies, offering boys who survive childhood cancer the option of having biological children of their own in the future.
References
1. Khattak H, Malhas R, Craciunas L, et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: a systematic review and individual patient data meta-analysis. Human Reproduction Update 2022;28:400-16.
2. Braye A, Delgouffe E, Van Der Werff Ten Bosch J, et al. Gonadal development and function after immature testicular tissue banking as part of high-risk gonadotoxic treatment. Pediatr Blood Cancer 2023;E30370.
3. Fayomi AP, Peters K, Sukhwani M, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019;363:1314-9.
4. Yokonishi T, Sato T, Komeya M, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun 2014;5:4320.
5. Rimmer MP, Howie RA, Subramanian V, et al. Outcome reporting across randomised controlled trials evaluating potential treatments for male infertility: a systematic review. Human Reproduction Open 2022;2022(2):hoac010.
Declaration of competing interests:
Michael Rimmer: Research Funding: The Urology Foundation, The Barbour Watson Trust & Medical Research Council, The Moray Fund - University of Edinburgh.
Kathleen Duffin: Research Funding: CRUK.
Rod Mitchell: Research Funding: UKRI Future Leaders Fellowship, NIHR, European Society for Human Reproduction and Embryology, Children with Cancer UK.