Infertility is the inability to achieve pregnancy following one year of regular, unprotected intercourse (in the fertile phase of the menstrual cycle). Infertility is a common problem that affects between 7 and 15% of couples worldwide, with male factor infertility responsible for up to 40% of all cases [1]. The inability to have children still carries a significant stigma.
The human body is engineered such that male and female gametes ‘meet’ during fertilisation under precise conditions to form a life form (the zygote) which then develops into an embryo. For fertilisation to occur, millions of sperm cells are ejaculated, usually at the peak of sexual activity, by the male partner into the female reproductive tract. These sperm cells must swim up the vaginal and uterine cavities into the oviduct to interact with an oocyte released by the ovary [2]. The major aim of assisted reproductive technology (ART) is to mimic the natural process by providing an artificial environment suitable for fertilisation to occur [3].
Certain physiological barriers ensure the selection of the spermatozoon with the highest fertilisation potential and capacity to achieve optimal embryonic and foetal development. This selection process is highly dependent on the morphology and other dynamic characteristics of the sperm. Sociodemographic trends have fuelled interest in optimising ART outcomes, with a recent male focus exploring various sperm selection techniques (SST) aimed at imitating the natural process.
History of ART
Assisted reproduction techniques have been practised for millennia. In the seventh century BC, the ancient Greeks believed in infertility treatments based on religious superstitions and magic; this later gave way to scientific reasoning following Hippocrates’ formulation of ‘the humoral theory’. Infertility was recognised as a medical problem that required a diagnosis and treatment [2].
In the ancient era (3500 BC – 500 AD), Vedic civilisations were familiar with the concept of infertility and ‘assisted reproduction’. Sages made ‘magic potions’ for the wives of childless kings to help them conceive. The Vedic literature also describes artificial insemination (AI) which involves the manual injection of semen into the female reproductive tract [2].
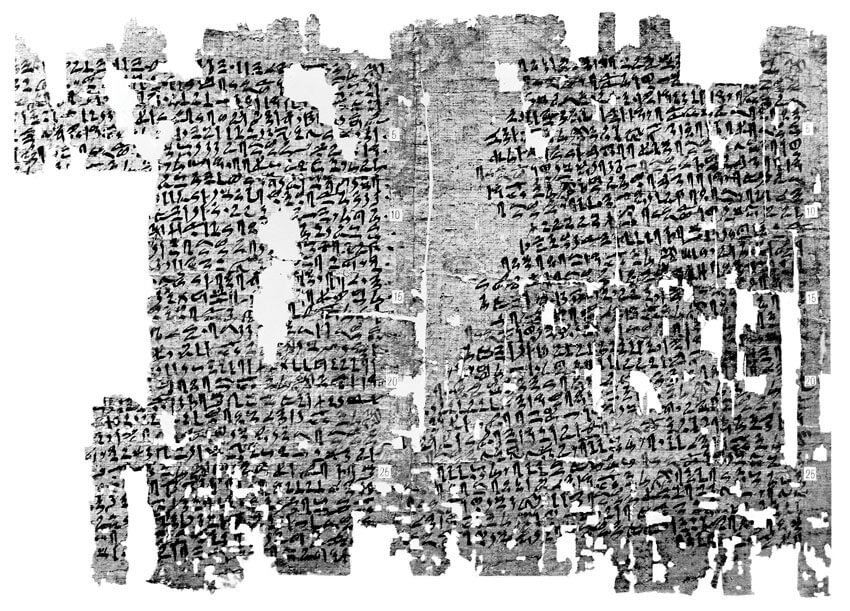
Figure 1: The Kahun Gynaecological Papyrus (Wellcome Library, London).
The Kahun Gynaecological Papyrus (~1800 BC) is the oldest known medical text of the ancient Egyptian era and describes various tests for fertility and practices both to assist conception as well as for contraception (Figure 1). Pregnancy was diagnosed based on the ability of the woman’s urine to grow cereals or her aversion to strong odours. Amulets, potions and rituals were use to assist childless women to conceive [2].
The Middle Ages and Renaissance saw significant development in medicine, including infertility treatment. This period also saw the rise of religious institutions in Europe. To treat infertility, various remedies and potions were combined with prayers or spells, the practice of penance, and pilgrimages. The Middle Ages also saw the emergence of Islamic scholars in the Middle East. The prominent scholar Ibn Sina (Avicenna) authored the encyclopaedic Al-Qanun fit-Tibb (the Canon of Medicine), which detailed theories on the treatment of diseases, including infertility. It became the major medical text both in the Islamic world and Europe until the 18th century [2].
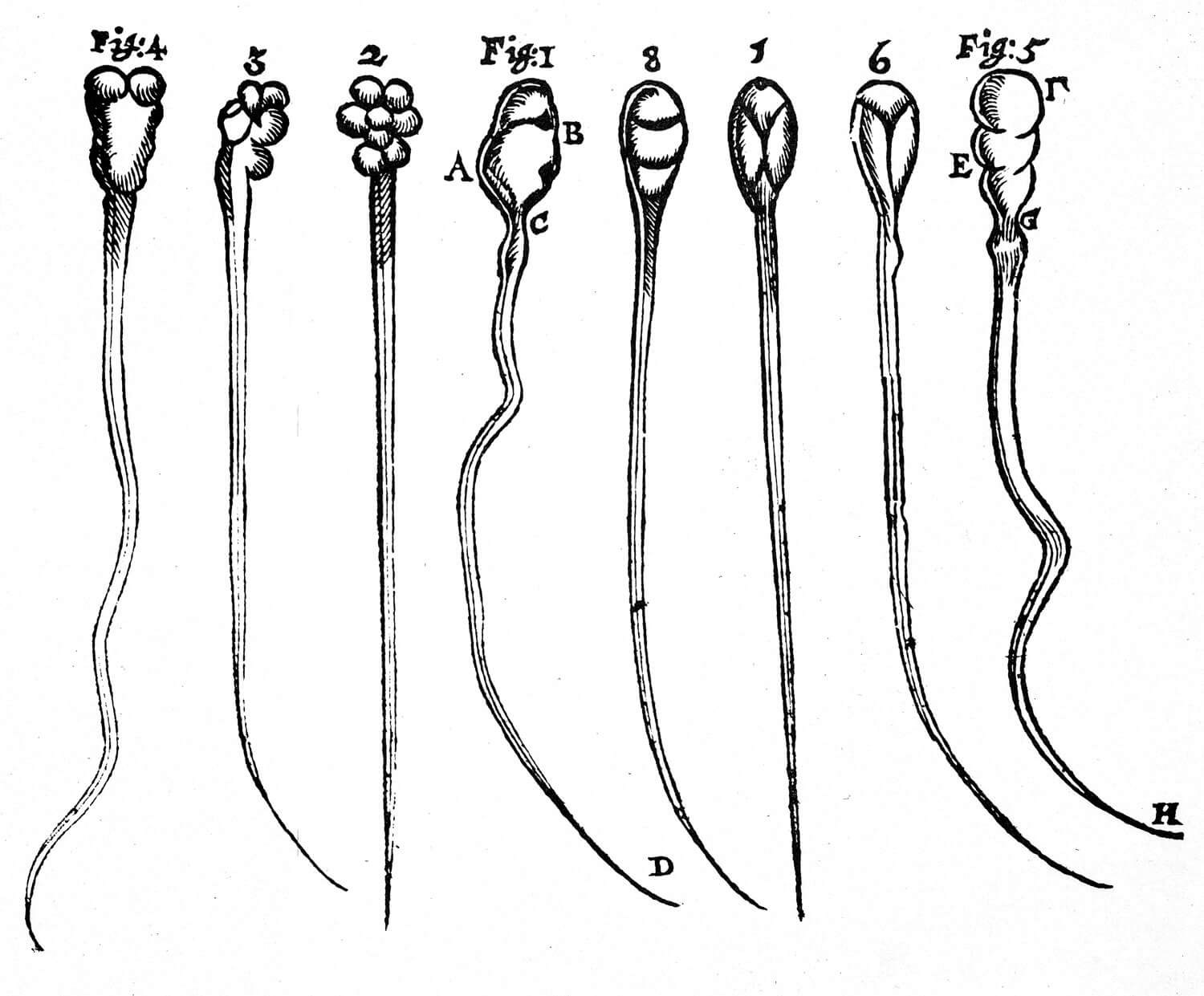
Figure 2: Antoni van Leeuwenhoek’s concept of spermatozoon (figs 1-4: rabbits, figs 5-8: dogs).
Scientific breakthroughs in the late 1600s laid the foundation for modern ART. Antonie van Leeuwenhoek discovered spermatozoa using the microscope in 1677 (Figure 2). In 1779, Italian priest and physiologist Lazzaro Spallanzani demonstrated that spermatozoa are essential for fertilisation when he successfully inseminated dogs. The first documented AI was reported by John Hunter in 1790; the semen of a cloth merchant with severe hypospadias was collected in a warmed syringe and used to artificially inseminate his wife. Karl Ernst Von Baer discovered the mammalian ovum in 1827 [2].
The advent of in-vitro fertilisation (IVF) marked a significant upsurge in ART. Walter Heape was the first to record IVF and embryo transplantation in rabbits at Cambridge in 1890. Eight decades later, Robert Edwards and Patrick Steptoe carried out the first successful IVF in a human, resulting in the birth of the world’s first ‘test tube baby’ (Louise Brown) in 1978 [2,4].
Fertilisation of an oocyte using a single sperm injection was first achieved in sea urchins, mice, and hamster models in the 1960-70s. However, it took a further 20 years to achieve live offspring from sperm injection into oocytes in other mammalian models; success was achieved in rabbits in 1988, and bovine species in 1990. In humans, Lanzendorf reported on successful microinjection of a spermatozoon into oocytes in 11 patients in 1987, although no zygotes were transferred. The technique was further developed and standardised over the next few years. In 1992, Palermo et al. described that, whilst performing subzonal insemination (SUZI), the oolemma and ooplasm of a human oocyte were inadvertently pierced with a single spermatozoon; when examined the next day, two pronuclei were noted, confirming fertilisation. This was, perhaps, the advent of modern intracytoplasmic sperm injection (ICSI) [5].
Current trends
ART techniques
Following the birth of the world’s first ‘test tube baby’ from IVF in 1978, alternative ART techniques have been developed and refined with many in use today (e.g. ICSI, gamete intrafallopian transfer (GIFT), zygote intra-fallopian transfer (ZIFT), pronucleate transfer (PROT), assisted hatching, round nuclei injection or spermatid injection). ICSI has replaced IVF as the most used ART, especially in the developed world (Figure 3) [3].
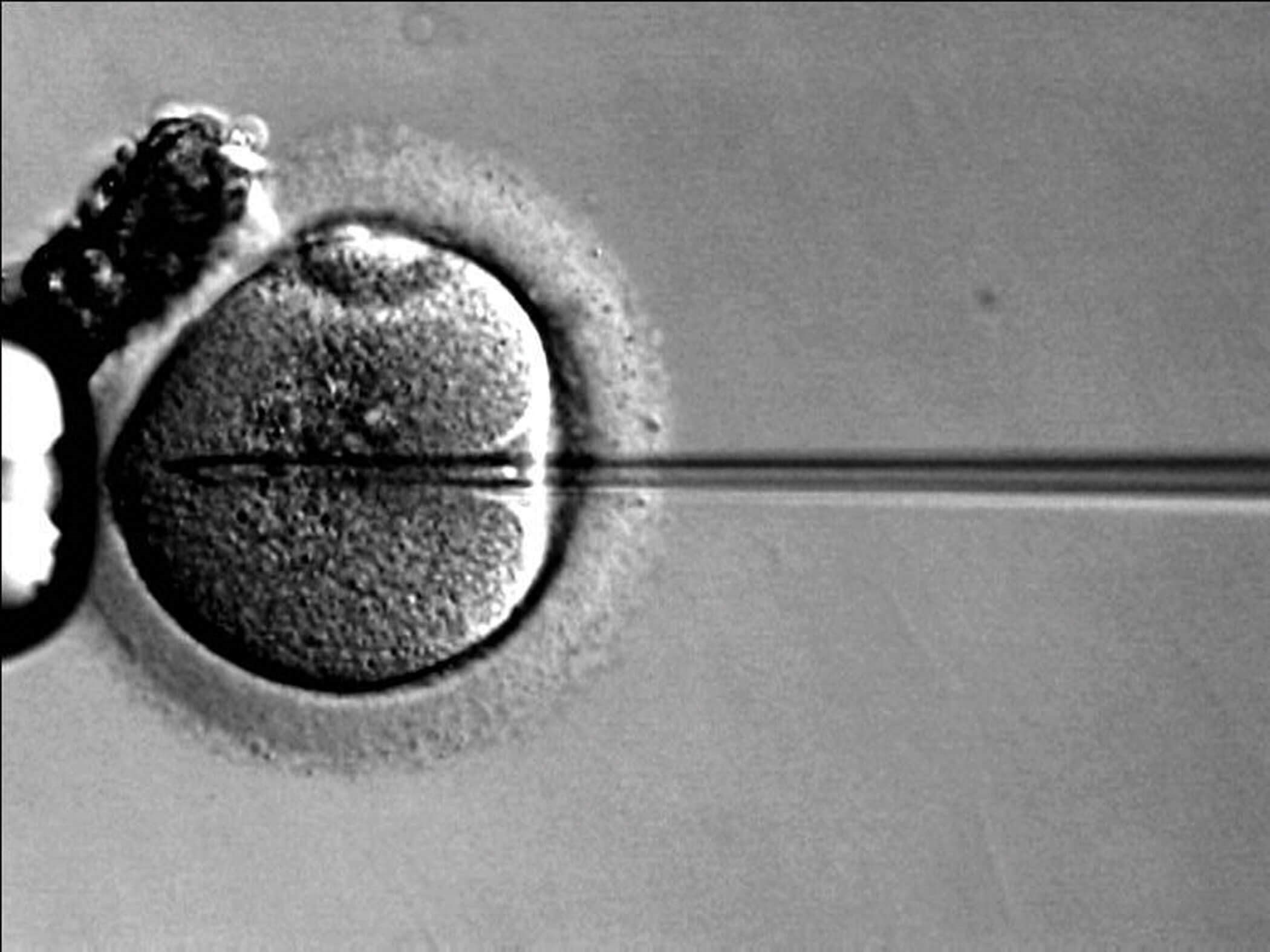
Figure 3: Intracytoplasmic sperm injection into a single oocyte.
Sperm DNA fragmentation
Traditionally, spermatozoa for use in ART are selected based on several semen parameters. However, ‘conventional’ semen analysis may provide inadequate assessment of the male fertilisation potential. The quality of, rather than the number and motility of, spermatozoa has been found to be a more accurate predictor of male fertility. Therefore, the evaluation of the quality of the genetic material (i.e. deoxyribonucleic acid (DNA)) in sperm cells is currently a valuable tool to assess male infertility, with sperm DNA fragmentation (SDF) being used as a marker [6,7]. Meta-analysis has shown that high SDF may negatively affect ART outcomes, resulting in lower pregnancy and higher miscarriage rates [8].
Damage to sperm DNA can be in the form of breakage into fragments, defects in the genetic material, abnormalities with nuclear maturity, and chromosomal disorders. Although these may be caused by intrinsic factors (abnormality in spermiogenesis during DNA synthesis), extrinsic factors play a bigger role and include damage secondary to acute and chronic infections, chemotherapy, radiotherapy, diabetes mellitus, obesity, cigarette smoking, drug use, advanced age, and elevated reactive oxygen species level. The prevention of DNA damage focuses on the management of the underlying causes (i.e. treating any infections, lifestyle modifications including cessation of smoking and alcohol, abstaining from prolonged exposure to extremes of temperature, and varicocele repair). Should infertility persist, patients are usually offered ART as an alternative to natural conception [7].
There are different techniques used to evaluate the degree of SDF. The diagnostic accuracy of these techniques depends on many biological and technical aspects. Commonly used tests include sperm chromatin structure assay (SCSA), acridine orange test (AOT), terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling assay (TUNEL) and single-cell electrophoresis (COMET) assay, which assess the integrity of sperm DNA. Other tests (e.g. aniline blue staining, chromomycin A3 staining and toluidine blue staining) detect defects in the packaging of sperm chromatin. It remains unknown which assay technique is the most reliable for assessing SDF [7,9].
Sperm selection techniques (SST)
The aim of sperm selection is to ‘mimic’ the natural environment and physiological reactions that occur prior to fertilisation. Although millions of sperm cells are released during ejaculation, only the spermatozoon with the highest fertilisation potential and capacity to achieve optimal embryonic and foetal development reaches the oviduct. The acidity of the vagina, the viscosity of the cervical mucus, the different changes that occur within the uterus and the oviducts, and the sperm-oocyte (zona pellucida) reaction all ensure the elimination of physiologically abnormal spermatozoa. These mechanisms effectively select only a fraction of the ejaculated sperm cells that reach the oocyte to fertilise it. Moreover, the process allows the sperm cell to reach optimal capacitation via the ‘acrosomal reaction’ which occurs following interactions with chemoattractant molecules within the female reproductive tract. This helps modulate the interaction between the sperm cell and oocyte and ultimately leads to fertilisation and the formation of a blastocyst (and then an embryo). Hence, only the sperm with the best fertilisation potential is selected [3,10].
The ideal sperm selection technique should be ‘simple, cheap and fast,’ and should also be highly efficient such that it differentiates morphologically normal and motile sperm cells from other cell types. Novel methods have now been developed which help to identify the ‘best’ spermatozoon; motile, morphologically normal, viable with intact membranes and high levels of DNA integrity. Commonly used methods of selection include physiological intracytoplasmic sperm injection (PICSI), intracytoplasmic morphologically selected sperm injection (IMSI) and magnetic-activated cell sorting (MACS) [11].
In PICSI, sperm is selected based on the specialised receptors (hyaluronic acid and zona pellucida) which are found only on the plasma membranes of mature morphologically normal sperm cells. These receptors aid in the acrosomal reaction. Immature sperm or those with damage to their DNA have defective receptors [12]. During IMSI, sperm cells are selected based on careful examination under high-powered magnification. Only the morphologically normal sperm cells are selected for use. It has been suggested that having a standardised grading system for sperm morphology will improve the fertility outcomes seen with IMSI [4,12]. During MACS, apoptotic sperm cells are labelled using magnetic nanoparticles. The sample is then passed via a magnetic filter. This reduces the proportion of sperm with high DNA fragmentation, improving the fertilisation potential and outcomes [11,12].
Perhaps due to their novelty, there is limited evidence for PICSI, IMSI and MACS. The Human Fertilisation and Embryology Authority (HFEA) rates both PICSI and IMSI as ‘red’ (i.e. insufficient supporting evidence to support routine use), and a recent Cochrane review of SSTs noted the lack of high-quality studies [13]. Nevertheless, emerging evidence suggests SSTs may have a role in men with high SDF; in their 2022 randomised controlled trial, Hozyen et al. report that PICSI and MACS both provide significant improvement in embryological and clinical outcomes over testicular sperm alone [14].
Other lesser-known techniques being used for sperm selection in ICSI include the hypo-osmotic swelling test (HOST) and microfluidics (e.g. ZyMot). HOST assesses the functional integrity of the sperm membrane. In this test, sperm is exposed to a hypo-osmotic fluid; the tails of healthy sperm will swell. Microfluidics considers the biochemical environment in which the sperm cells interact in the female reproductive tract. Sperm cells are sorted through micro-barriers, thereby isolating the most motile, morphologically normal sperm. This system has been shown to increase the percentage motility of the sorted sperm [3,11,12]. Although promising, these techniques are in their infancy, and high-quality evidence is sparse.
Where do we go from here?
Currently, uptake of newer assisted-reproductive technologies is limited. There is lack of consensus on the accuracy and validity of the techniques used to evaluate sperm DNA quality. Additionally, despite some studies suggesting that SSTs may improve ART outcomes in patients with infertility, randomised prospective comparative evidence is limited; no individual selection technique stands out. Nevertheless, there is huge potential for innovation. When these barriers can be circumvented, we will undoubtedly see rapid progress.
References
1. Agarwal A, Cho C-L, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol 2016;28(3):164-71.
2. Sharma RS, Saxena R, Singh R. Infertility & assisted reproduction: A historical & modern scientific perspective. Indian J Med Res 2018;148(Suppl):S10-4.
3. Begum MR. Assisted reproductive technology: techniques and limitations. J Bangladesh Coll Phys Surg 2008;26:135-141.
4. Kushnir VA, Barad DH, Albertini DF et al. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol 2017;15(1):6.
5. Rosenwaks Z, Pereira N. The pioneering of intracytoplasmic sperm injection: historical perspectives. Reproduction 2017;154(6):F71-7.
6. Agarwal A, Zini A, Sigman M. Is sperm DNA integrity assessment useful? J Urol 2013;190(5):1645-7.
7. Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril 2005;84(4):850-3.
8. Deng C, Li T, Xie Y, et al. Sperm DNA fragmentation index influences assisted reproductive technology outcome: A systematic review and meta‐analysis combined with a retrospective cohort study. Andrologia 2019;51(6):e13263.
9. McDowell S, Kroon B, Ford E, et al.Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev 2014;10:CD010461.
10. Baldini D, Ferri D, Baldini GM, et al.Sperm Selection for ICSI: Do We Have a Winner? Cells 2021;10(12):3566.
11. Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod 2019;101(6):1076-82.
12. Malhotra K. Advances in Sperm Selection. In: Malhotra J, Malhotra N, Sharma DG. FOGSI FOCUS Emerging Trends in Infertility. New Delhi, India; Jaypee Brothers Medical Publishers (P) Ltd; 2018:15-8.
13. Lepine S, McDowell S, Searle LM, et al. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev 2019;7:CD010461.
14. Hozyen M, Hasanen E, Elqusi K, et al. Reproductive Outcomes of Different Sperm Selection Techniques for ICSI Patients with Abnormal Sperm DNA Fragmentation: a Randomized Controlled Trial. Reprod Sci 2022;29(1):220-8.
Declaration of competing interests: None declared.