Incorporation of family history (FH) status into prostate cancer (PCa) risk stratification has the potential to underpin many aspects of PCa care. This group of men presents a unique challenge in early cancer detection, particularly given that most men without additional risk factors have a low lifetime risk of developing lethal PCa and the diagnosis of low-grade, screen detected PCa often represents overdiagnosis and overtreatment.
Current translational research is focusing on how we can best stratify men into groups who will benefit from screening, and how to develop the best screening test(s) to offer them.
How common is the problem?
In the largest prostate specific antigen (PSA) screening studies (ERSPC and PLCO), rates of FH varied from approximately 6-7.5% [1,2].
How do we define FH?
Men with an FH of PCa have a significantly higher lifetime risk of developing the disease, with a two to eight fold increase reported [3], with worsening risk correlated with the number of first degree relatives (FDR) affected. In men with familial PCa, this is due to rare, moderate to high penetrance mutations and the presence of multiple, common low penetrance alleles in the germline, with men carrying mutations in the breast cancer gene (BRCA) and other DNA repair genes at particularly high risk.
Risk of PCa also varies due to strength of family history e.g., first degree or second-degree relative, as well as the age of PCa onset in family members. The terms ‘family history’, ‘familial prostate cancer’ and ‘hereditary prostate cancer’ (HPC) are not synonymous. Conflicting evidence exists regarding treatment outcomes, survival and grade / stage of disease in these men compared to men without an FH.
Degree of family history
There is variation in the reporting of FH. Some studies report it simply as a ‘yes’ or ‘no’ indicating presence or absence with no further information on number of relatives or the degree. In a single-centre retrospective assessment of over 4000 men in the Cleveland clinic, men undergoing prostate biopsy following a raised PSA or abnormal digital rectal examination (DRE), a significantly higher incidence of PCa (all grades) of prostate cancer was found in men with an FH (54%) compared to those without (45%) with an OR of 1.6 [4]. In this setting, FH was defined as “at least one first-degree relative (FDR) with PCa (at any age)”.
A large amount of evidence relating to familial PCa comes from The Swedish Family Cancer database. The highest hazard ratio (HR) for being diagnosed with PCa was reported for younger men (<65 years) with three brothers affected with PCa and the HR increased as the age of onset of disease in a father or brother decreased. This study did not differentiate between aggressive or low-risk disease at diagnosis but did also examine risk of death from PCa i.e. lethal / aggressive PCa. They reported a HR of 6.3 in men with two affected brothers [5,6].
Twins
A Scandinavian twin study described a large effect of the heritability in PCa in a study of over 40,000 pairs of both monozygotic (identical) and dizygotic (non-identical) twins. They found a significantly higher absolute risk of PCa in a man with either an identical (18%) or non-identical twin (3%) with the disease and showed that the difference in age of onset of PCa was shorter in men with an identical twin (5.7 years) with PCa than in men with a non-identical twin (8.8 years) [7]. A more recent study prospectively followed-up over 200,000 twins across Norway, Sweden, Denmark and Finland over 32 years for the development of incident cancers. Significant heritability for PCa of 57% was noted (95% CI 51%-63%) [8].
Screening effect in the PSA era
The likelihood for increased PSA activity (i.e. screening) in men with a recently diagnosed relative is worth bearing in mind when considering the effect of FH status on PCa risk. For example, the magnitude of risk associated with having an FH of PCa and an increased incidence of the disease observed in relatives could be in part due to increased PSA screening and detection of low-risk, early detected disease. Blatt et al., recognising this issue, reported on age-specific probabilities of PCa stratified by the number of affected relatives, their age at diagnosis and the risk category of the affected relative’s cancer [9].
Is FH always due to an underlying germline genetic pathogenic variant?
Both monogenic and polygenic causes for PCa exist, together explaining approximately 34% of familial disease. Mutations in HOXB13 have been linked specifically to familial and HPC. Mutations in genes involved in DNA and mismatch repair such as BRCA1/2, ATM and CHEK2 have been associated with an increased risk of developing PCa in men unselected for FH as well as in men with familial PCa.
In an analysis of a subset of men with an FH of PCa in the United Kingdom Genetics Prostate Cancer Study (UKGPCS) [10], 7.3% of patients with a positive FH were found to carry a pathogenic germline variant. The most frequent pathogenic variant was in BRCA2 (28.57%), and importantly there was a significant association between genetic mutation carrier status and nodal and metastatic disease [10].
How has PSA performed in this population?
A subset analysis of ERSPC (n=4932) analysed the effect of FH in the Swiss cohort. Cumulative, screen-detected PCa incidence over 11 years was reported as significantly different between men with and without an FH (18% vs. 12% respectively; HR 1.6). Median PSA at cancer diagnosis was 4.7-4.5ng/ml and mean age was 66-67 years (positive FH vs. negative FH respectively). They reported FH along with age and baseline PSA as significant predictors of overall PCa incidence, but only baseline PSA acted as an independent predictor for Gleason ≥7 cancer [1]. When men were stratified by FH status alone, 5.1% of men with an FH of PCa were found to have clinically significant cancer compared to 4% of men without an FH (no statistically significant difference).
The PLCO trial data has also been interrogated for PCa incidence and mortality differences in men with and without an FH. Similar to ERSPC data, an FH of PCa was associated with a higher probability of cancer diagnosis (HR 1.59) with the number of affected FDRs correlating positively with risk. By FH status (one FDR with PCa), 10.5% of men without an FH were found to have PCa compared with 16.5% of men with an FH. There was no difference in cancer stage, age or PSA at diagnosis between the groups nor was there any effect on mortality from PCa over both interventional and non-screening arms. When analysis was repeated by screening / interventional arm vs. non-interventional arm, FH in an FDR and the number of FDRs was significantly associated with PCa mortality (HR 1.89) in the non-screening arm compared to the interventional arm [2] suggesting a benefit to screening this group.
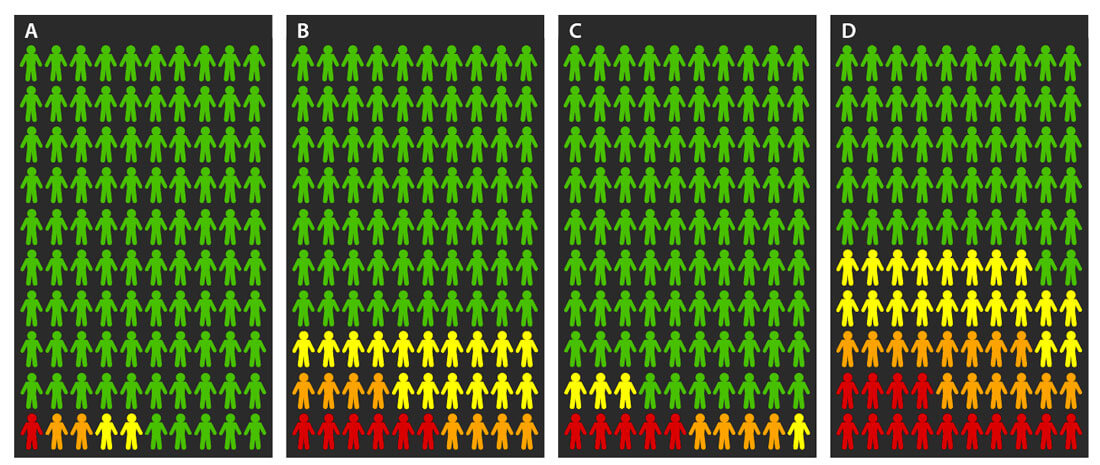
Figure 1: Pictograms of the probabilities of no risk (green), low-risk (yellow), intermediate risk (orange), and high-risk, including metastatic (red) prostate cancer. A) Average population probabilities at age 65 years. B) Probabilities at age 65 years for men with a father and one brother with prostate cancer. C) Average population probabilities at age 75 years. D) Probabilities at age 75 years for men with a father and one brother with prostate cancer. Created based on data from Bratt et al. [9].
FH analyses in the placebo arms of the PCPT and REDUCE trials
PCPT investigated the use of finasteride, a five-alpha reductase inhibitor (5ARI) in PCa prevention. In the placebo arm of the study, men either underwent end of study biopsy (at seven years) or a clinically recommended biopsy if PSA was ≥4.0ng/ml or abnormal DRE at any of the men’s annual study visits up to year seven [11].
In an analysis of 5519 men in the placebo arm of this study, men with an FH (16% of the cohort) of PCa had an odds ratio (OR) of 1.31 for harbouring PCa on any form of biopsy throughout study follow-up. The median PSA of this cohort at study entry was 1.5ng/ml and 88% of men had a PSA ≤4.0ng/ml. Approximately 24% of men with an FH who underwent prostate biopsy had (any grade) PCa compared with 17% of men without an FH. FH was not associated independently with high-grade disease. Approximately 95% of this cohort was Caucasian [12].
A sub-analysis of the REDUCE study also examined the effect of FH on PCa incidence at time of biopsy in both treatment and placebo arms of this (dutasteride vs. placebo) RCT. In the placebo arm, they found PCa (all grades) in 23% of men undergoing biopsy with an FH compared to those without (19%) in the placebo arm, and a 31% risk reduction (RR) in PCa with dutasteride [13,14].
In summary, large scale PSA-based screening / interventional studies have shown in those with an FH of PCa, PSA testing detected PCa in approximately 16-18%. The overall proportion of these cancers which are clinically significant, or Gleason ≥7, seems to be similar between men with and without an FH (28.3% vs. 33.3%; P=0.3 respectively in the Swiss cohort of ERSPC).
Is the prognosis different?
Evidence is conflicting regarding if a true difference in outcomes exists between men with and without an FH with some authors citing unfavourable histology and inferior outcomes in men with an FH. Early post-prostatectomy studies by Kupelin et al. showed a difference in biochemical recurrence (BCR) rates between men with and without an FH [15,16]. There have also been reports of a higher incidence of lethal PCa in men with an FH in first-degree relatives (FDRs) [17]. Ultimately not enough prospective data exists regarding the long-term follow-up and clinical outcomes in men across all treatment modalities with an FH of PCa.
Future screening strategies in men with an FH
Both the ERSPC and PLCO trials have so far failed to convince the urological communities of the certain mortality benefits of PSA screening and the US Preventative Services Task Force (USPSTF) currently does not recommend routine PSA screening for PCa but acknowledges a benefit may exist for black men and men with an FH of PCa.
For the general population, PSA screening may predominantly detect indolent or clinically insignificant prostate cancer, having little impact on mortality and exposing men to the physical and psychological morbidity of diagnostic procedures and potentially unnecessary treatments. Developing tailored, specific risk-based screening protocols and diagnostic pathways and targeting them to only higher-risk groups of men would allow less low or average-risk men to be exposed to the harms of overdiagnosis and overtreatment.
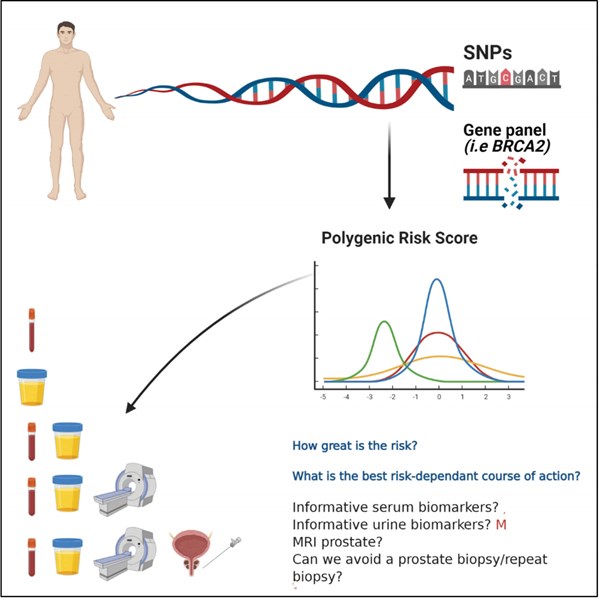
Figure 2: Infographic demonstrating a potential future targeted assessment
of a man with a FH of PrCa to help inform screening decisions.
Germline single nucleotide polymorphisms (SNPs)
Common alleles associated with PCa exist. These low-penetrance genetic variations may confer a low risk of PCa if occurring alone but result in an elevated risk when multiple variations occur together, strengthening their genetic effect. The rollout of large scale genome-wide association studies (GWAS), has led to the discovery of up to 170 SNPs associated with prostate cancer risk across various chromosomal loci [18,19]. At present, men in the top 1% of the risk profile have a 4.7-fold increase in risk of developing PCa compared to controls [20,21]. Zheng and colleagues examined the effect of five SNPs known to be associated with PCa. They found their presence in combination with an FH accounted for 46% of the cases of PCa in their cohort and conferred an OR of 9.46 compared to men who had none of these factors, independent of PSA [19].
The construction of polygenic risk scores (PRS) using pathogenic SNPs for defining PCa risk, in addition to clinical information, has been shown to potentially reduce the need for prostate biopsies and accurately predict PCa [22,23]. It is likely in the future, as more pathogenic SNPs are discovered, that the usefulness and performance of PRS will improve. Pashayan et al. assessed the implications of using PRS on reducing overdiagnosis. They constructed a PRS on 17,000 men aged 50-69 from three large studies (ProtecT, SEARCH and UKGPCS) using 66 known PCa risk SNPs, separating men with and without cancer into risk quartiles. By using this method, they found a 56% reduction in overdiagnosis between the lowest risk quartile and the highest [24].
Recently, Leeuwen et al. created a prediction model to determine risk of clinically significant PCa, adding mpMRI PiRADS score to clinical data. Applying this to 198 men and using their nomogram risk of ≥10% as a biopsy threshold would mean 28% of biopsies were avoided missing 2.6% of clinically significant cancers [25].
Studies investigating targeted screening in men with a suspected PCa predisposition
Currently, the IMPACT study has enrolled over 3000 cases and controls to investigate the role of targeted PSA screening in men with BRCA1/2 mutations and Lynch Syndrome. Early results have suggested a screening strategy in this population is beneficial [26]. Led by the Institute of Cancer Research, the UKGPCS will recruit 26,000 men with a diagnosis of prostate cancer, including those with diagnosis at a young age and those with an FH in order to map the genetic changes associated with the disease in these cohorts [27-29].
The PROFILE Feasibility Study examined the role of upfront prostate biopsy regardless of PSA with a PRS in 100 men. They reported a cancer detection rate of 25%, with 48% of these being clinically significant cancers requiring radical treatment [30]. Presently, the PROFILE study (NCT02543905) is recruiting a total of 700 subjects investigating the role of targeted screening in men with an FH of PCa and in black men. Germline genetic analysis of 130 SNPs with a resulting PRS will be correlated with outcome at upfront prostate biopsy. The aim is to recruit 350 men in each risk-group, with men declining MRI / biopsy undergoing PSA surveillance for a minimum of five years, with low-age related thresholds set for clinically ‘triggering’ a prostate biopsy. PCa incidence, aggressiveness and the incidence of abnormal MRI and its value in this cohort will also be investigated. This study will aim to map the incidence of PCa in this population both on upfront testing and throughout long-term follow-up and aims to investigate if a genetic profile can predict outcome at prostate biopsy [30].
Current guidelines
American guidelines suggest individualised screening following shared decision-making using PSA in men aged 40+, in men who have an FH of lethal adenocarcinomas spanning multiple generations, in multiple FRs and at young age of onset [31]. The most recent European Association of Urology (EAU) guidance (2021) cites strong evidence for offering PSA screening in men from age 45 with an FH of PCa.
Conclusion
It has been established that FH can predispose men to earlier onset of PCa. We know PSA is an imperfect test, and the basis for why this group of men are at higher risk is suspected to be genetic in origin. It therefore seems sensible to develop targeted screening strategies for this cohort, using a combination of screening and diagnostic tools for early cancer detection.
Advances in the field of urology including the diagnostic performance of mpMRI and genomic interrogation mean our screening and diagnostic tests may be used more intelligently. The future of screening in men with an FH of PCa may involve early identification, germline genetic testing to rule out a hereditary cancer predisposition syndrome, further stratify based on germline status using risk SNPs and tailor further intervention (i.e. MRI / biopsy) based on individual risk profile.
References
1. Randazzo M, et al. A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau). BJU Int 2016;117(4):576‑83.
2. Abdel-Rahman O. Prostate Cancer Incidence and Mortality in Relationship to Family History of Prostate Cancer; Findings From The PLCO Trial. Clinical Genitourinary Cancer 2019;17(4):e837-e844.
3. Goldgar DE, et al. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 1994;86(21):1600-8.
4. Elshafei A, et al. Does positive family history of prostate cancer increase the risk of prostate cancer on initial prostate biopsy? Urology 2013;81(4):826-30.
5. Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer 2001;92(1):144-50.
6. Hemminki K, Czene K. Age specific and attributable risks of familial prostate carcinoma from the family-cancer database. Cancer 2002;95(6):1346-53.
7. Lichtenstein P, et al. Environmental and heritable factors in the causation of cancer - analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343(2):78-85.
8. Mucci LA, et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016;315(1):68‑76.
9. Bratt O, et al. Family History and Probability of Prostate Cancer, Differentiated by Risk Category: A Nationwide Population-Based Study. J Natl Cancer Inst 2016;108(10):djw110.
10. Leongamornlert D, et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br J Cancer 2014;110(6):1663-72.
11. Thompson IM, et al. The Influence of Finasteride on the Development of Prostate Cancer. New England Journal of Medicine 2003;349(3):215-24.
12. Thompson IM, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98(8):529-34.
13. Andriole GL, et al. Effect of Dutasteride on the Risk of Prostate Cancer. New England Journal of Medicine 2010;362(13):1192-202.
14. Tammela TLJ, et al. The Influence Of A Positive Family History On Prostate Cancer Incidence And Dutasteride Efficacy In The Reduce Study. European Urology Supplements 2010;9(2):301.
15. Kupelian PA, et al. Aggressiveness of familial prostate cancer. J Clin Oncol 2006;24(21):3445-50.
16. Kupelian PA, et al. Familial prostate cancer: a different disease? J Urol 1997;158(6):2197‑201.
17. Rodrigue, C, et al. Family history and risk of fatal prostate cancer. Epidemiology 1997;8(6):653-7.
18. Macinnis RJ, et al. A risk prediction algorithm based on family history and common genetic variants: application to prostate cancer with potential clinical impact. Genet Epidemiol 2011;35(6):549-56.
19. Zheng SL, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med 2008;358(9):910-19.
20. Schumacher FR, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet 2018;50(7):928-36.
21. Benafif S, et al. A Review of Prostate Cancer Genome-Wide Association Studies (GWAS). Cancer Epidemiol Biomarkers Prev 2018;27(8):845-57.
22. Aly M, et al. Polygenic risk score improves prostate cancer risk prediction: results from the Stockholm-1 cohort study. Eur Urol 2011;60(1):21-8.
23. Szulkin R, et al. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate 2015;75(13):1467-74.
24. Pashayan N, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med 2015;17(10):789-95.
25. van Leeuwen PJ, et al. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int 2017;120(6):774-81.
26. Page EC, et al. Interim Results from the IMPACT Study: Evidence for Prostate-specific Antigen Screening in BRCA2 Mutation Carriers. Eur Urol 2019;76(6):831-42.
27. Eeles RA, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet 2009;41(10):1116-21.
28. Eeles RA, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 2008;40(3):316-21.
29. Kote-Jarai Z, et al. Multiple novel prostate cancer predisposition loci confirmed by an international study: the PRACTICAL Consortium. Cancer Epidemiol Biomarkers Prev 2008;17(8):2052-61.
30. Castro E, et al. The PROFILE Feasibility Study: Targeted Screening of Men With a Family History of Prostate Cancer. Oncologist 2016;21(6):716-22.
31. Carter HB, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190(2):419‑26.
Declaration of competing interests: None declared.








