Traditionally, radical nephrectomy was the preferred operation for kidney cancer, while partial nephrectomy was reserved for specific circumstances and essential indications such as a tumour in a solitary kidney, bilateral kidney tumours, or severe chronic kidney disease (CKD). Given the increased and widespread use of imaging the majority of renal tumours are incidental findings and are small in size (<4cm diameter).
Following studies on tumour characteristics, it was found that at least 20% of tumours are benign. Furthermore, radical nephrectomy, which was widely used at the time, was associated with worse outcomes when compared to partial nephrectomy in terms of the onset of CKD, cardiovascular disease and overall survival. Given these findings, partial nephrectomy came into favour and is now recommended by the European Association of Urology (EAU) for small tumours (<4cm).
Partial nephrectomy is, however, no longer limited to only small tumours. Increasing surgical experience, and the influence of improved technology has allowed urologists to expand the use of partial nephrectomy to larger and more complex tumours, with good results. When considering larger tumours, radical nephrectomy may be a better option if it is not feasible to leave behind at least 40-50% of the kidney. In the most complex of cases with essential indications, for example a solitary kidney, bench surgery with renal autotransplantation is a last resort. Bench surgery is a long operation with considerable cold ischaemia time; hence it requires a multidisciplinary team within the operating room.
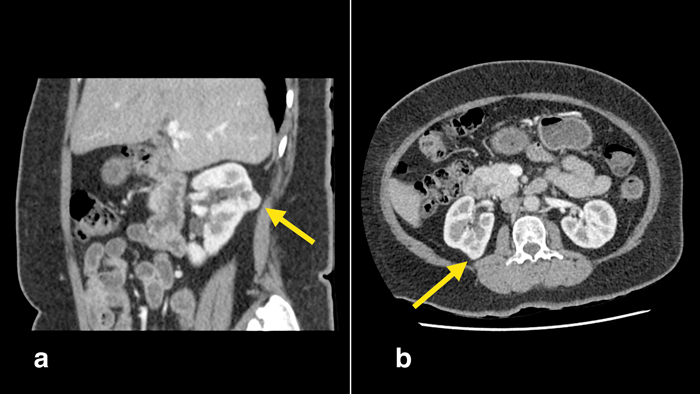
Figure 1: a) Sagittal and b) Axial CT scan to show a small posterior renal tumour (yellow arrows).
Assessment of tumours
Tumours are assessed with preoperative imaging. Modern computed tomography (CT) (Figure 1) and magnetic Resonance Imaging (MRI) scanners provide an excellent amount of detail allowing for accurate assessment of tumour location, size and metastases where applicable. These are key parameters used in the assessment of the tumours. Two scoring systems have been devised to assess tumours; the radius, endophytic / exophytic, nearness to collecting sinus anterior / posterior, location relative to polar lines (RENAL) nephrometry score and PADUA score.
A nephrometry score of <6 indicates low complexity, 7-9 is moderate complexity and >10 is highcomplexity. The PADUA score explores similar parameters (location, exophytic / endophytic, renal rim, involvement of renal sinus, involvement of urinary collection system, and size) with scores of 6-7 being low risk, 8-9 being intermediate risk and 10+ being high risk. This generally correlates difficulty of surgery with impact on warm ischaemia time (WIT) and length of operation. Tumours of larger size, tumours that are endophytic, and near the renal sinus are generally more difficult resections and they would hence score considerably higher than smaller exophytic tumours.
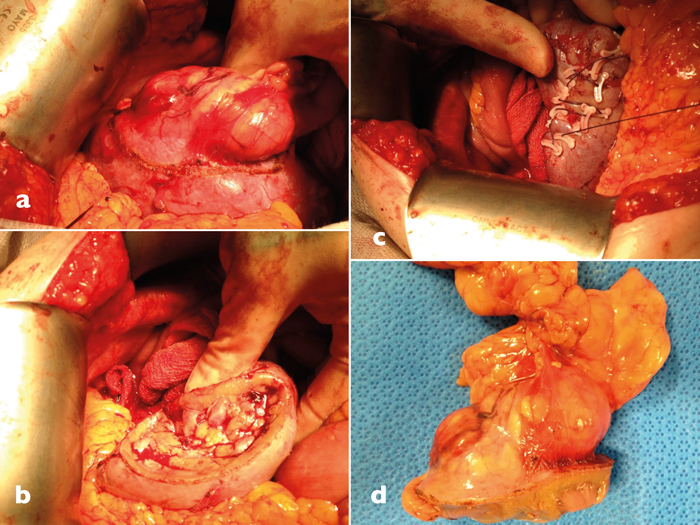
Figure 2: Open partial nephrectomy operation. a) Kidney defatted and tumour margin circumscribed with diathermy. b) Tumour crater following excision. c) Renorraphy with continuous vicryl and Hem-O-Lok clips. d) Surgical specimen.
Techniques
Open partial nephrectomy (OPN) (Figure 2) is generally associated with excellent outcomes. It is the standard procedure for centres that lack laparoscopic expertise and is often employed in complex situations such as a solitary kidney or higher risk procedures. It has a relatively low complication rate which include pain, herniation, urine leak and pulmonary embolism. Compared to laparoscopic partial nephrectomy (LPN), OPN has shorter operative times and shorter WIT. OPN, however, can be associated with increased blood loss, increased hospital length of stay, and increased pain compared to LPN. Complication rates are similar between the two types of procedure. LPN results in good postoperative outcomes comparable to open surgery, however, LPN has a steep learning curve. Outcomes of LPN are often operator-dependent with more experienced surgeons in centres with laparoscopic expertise producing better results along with shorter operative times.
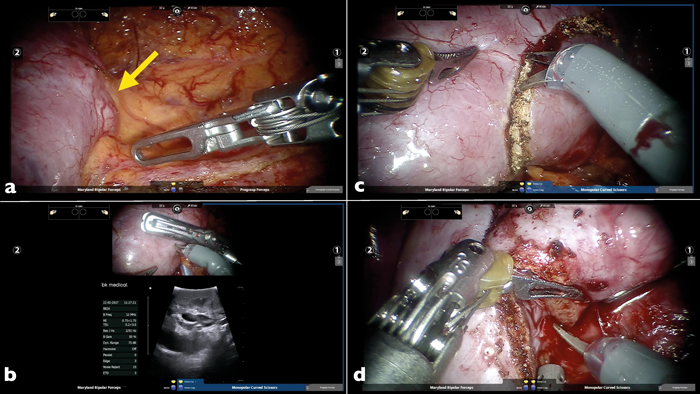
Figure 3: Robotic assisted partial nephrectomy operation. a) Kidney defatted and posterior hilar tumour just visible (yellow arrow). b) Intraoperative robotic ultrasound probe to delineate margin of tumour. c) Tumour margin circumscribed with diathermy. d) Tumour being excised with cold cutting and ‘fracturing’ of the healthy renal tissue.
Robot-assisted partial nephrectomy (RAPN) (Figure 3) utilises the four-arm da-Vinci robot (Intuitive Surgical Inc., Sunnyvale, CA, USA) with a camera port, an assistant port and two 8mm instrument ports. RAPN may be performed through the trans or retroperitoneal route. RAPN is gaining in popularity, and is, now, one of the fastest growing robotic operations in the world in terms of numbers. The robotic system provides 3D imaging and excellent wrist motion (EndoWrist, Intuitive Surgical Inc., Sunnyvale, CA, USA), allowing for better manoeuvrability, particularly in confined spaces. It offers the advantages of LPN while addressing some of the limitations of LPN. This includes a shorter learning curve for RAPN and the elimination of tremor. Furthermore, the robot also allows easier operation on more complex tumours that may be difficult to access or resect with the laparoscopic approach.
Recent studies comparing RAPN to OPN show similar advantages to that of LPN over OPN, which are less blood loss and shorter hospital stay. It additionally has fewer postoperative complications. However, there is increased WIT, and longer operative times [1]. Over LPN, RAPN offers advantages in WIT, blood loss and operating time [2]. Despite many of the advantages, the cost of purchasing and maintaining the robot is steep; hence RAPN is not easily accessible for all centres. Over time as RAPN further gains in popularity and with increased experience, it may see significant benefits over the other two techniques. Further studies are needed to assess long-term outcomes with RAPN.
Zero ischaemia
Standard partial nephrectomy (PN) involves a degree of warm ischaemia time with clamping of the renal artery to minimise bleeding during resection. The kidneys are, however, highly sensitive to ischaemia with longer WIT being associated with onset of CKD. Therefore, a key aim in nephron-sparing surgery is to reduce the WIT, despite the time limit that kidneys can tolerate warm ischaemia not being entirely clear. In select cases, selective clamping of branches of the renal artery is performed, or the renal artery is left unclamped altogether – the so called ‘off-clamp’ method. Currently, the off-clamp method is often reserved for smaller more peripheral tumours with lower R.E.N.A.L nephrometry and PADUA scores. Reports show a superior short-term outcome in postoperative renal function, lower positive margin rate and a lower postoperative complication rate for the off-clamp method. However, it is also associated with increased bleeding [3]. Long-term outcomes have yet to be explored, and further work in the form of randomised controlled trials is needed for better assessment.
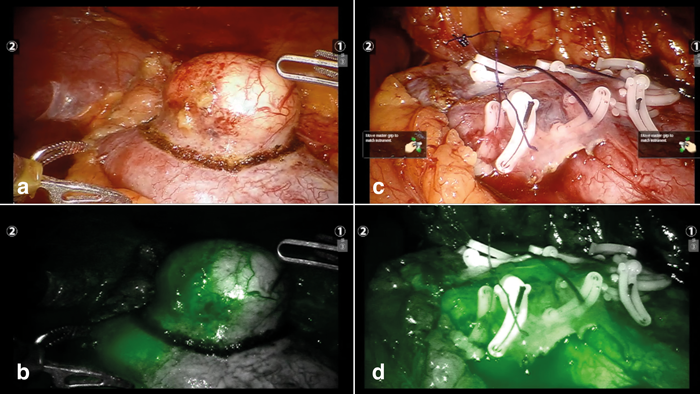
Figure 4: ICG to demonstrate perfusion of a kidney during RAPN. a) Tumour circumscribed, selective arterial clamp applied with infrared light OFF. b) Tumour circumscribed, selective arterial clamp applied with infrared light ON. There is only partial ischaemia of the tumour, therefore need to proceed with global ischaemia of the kidney. c) Renorraphy following excision of tumour in (a) with infrared light OFF. d) Renorraphy following excision of tumour in (a) with infrared light ON to demonstrate good perfusion of kidney on release of vascular clamp.
Intraoperative imaging
This is one of the more interesting developments for partial nephrectomy. LPN and RAPN often employ the use of intraoperative ultrasound (Figure 3b) to identify the tumour, corroborate pre-operative imaging and aid with surgical margins. New imaging methods are constantly being explored to aid surgery. Where selective vascular clamping is appropriate, indocyanine green (ICG) fluorescence imaging [4] has been used to identify tumour and aid selective arterial clamping (Figure 4). ICG is a dye that fluoresces bright green under infrared light. Normal tissue which fluoresces normally is distinguished from tumour which does not fluoresce well. Newer methods of identification such as fluorescence using molecular contrast agents (FRα) [5] have also been reported. Another more cost-effective method is the use of SonoVue bubble contrast-enhanced ultrasound to map renal blood flow and allowing for more accurate selective arterial clamping [6].
Since NHS England started to commission RAPN for small renal tumours unsuitable for LPN, RAPN has undergone a rapid growth spurt as a surgical treatment option for small kidney cancers. While at the present time oncological outcomes are similar to OPN, more widespread use of RAPN and the accumulation of experience with the robot may improve outcomes further. Shorter hospital stay and reduced blood loss are very significant benefits of RAPN. The impact of technology is also remarkable. A vast number of developments and expansion of knowledge with experience has allowed urologists to go from liberal use of radical nephrectomy, to the use of a robot for partial nephrectomy, assisted by intraoperative imaging, allowing for better tumour identification and more selective clamping. Currently there are not many studies comparing long-term outcomes and oncological outcomes given that many of these technologies are still relatively new, and only a few have seen widespread use. The field continues to grow at a rapid rate and the impact of technology is expected to improve surgical outcomes further.
References
1. Luciani LG, Chiodini S, Mattevi D, et al. Robotic-assisted partial nephrectomy provides better operative outcomes as compared to the laparoscopic and open approaches: results from a prospective cohort study. J Robot Surg [Epub ahead of print]
2. Wang Y, Ma X, Huang Q, et al. Comparison of robot-assisted and laparoscopic partial nephrectomy for complex renal tumours with a RENAL nephrectomy score>7: perioperative and oncological outcomes. BJU Int 2016;117(1):126-30.
3. Liu W, Li Y, Chen M, et al. Off-clamp versus complete hilar control partial nephrectomy for renal cell carcinoma: a systematic review and meta-analysis. J Endourol 2014;28(5):567-76.
4. Bjurlin MA, McClintock TR, Stifelman MD. Near-infrared fluorescence imaging with intraoperative administration of indocyanine green for robotic partial nephrectomy. Curr Urol Rep 2015;16(4):20.
5. Guzzo TJ, Jiang J, Keating J, et al. Intraoperative molecular diagnostic imaging can identify renal cell carcinoma. J Urol 2016;195(3):748-55.
6. Alenezi A, Motiwala A, Eves S, et al. Robotic assisted laparoscopic partial nephrectomy using contrast-enhanced ultrasound scan to map renal blood flow. Int J Med Robot 2017;13(1).
Declaration of competing interests: Mr Omer Karim is a proctor in robotic assisted surgery for Intuitive Surgical.