The diagnostic superiority of multiparametric magnetic resonance imaging (mpMRI) prior to targeted and systematic prostate biopsy over systematic transrectal ultrasound-guided (TRUS) biopsy alone in the detection of clinically significant prostate cancer (csPCa) has been proven by multiple level 1 studies [1].
When this is combined with the high reported negative predictor value (NPV) of prostate mpMRI it has resulted in a revolution in the established prostate cancer diagnostic pathway [2]. Routine pre-biopsy mpMRI in biopsy naïve men is now recommended in the most recent iterations of the European Association of Urology (EAU) and National Institute for Health and Care Excellence (NICE) prostate cancer diagnosis guidelines [3,4].
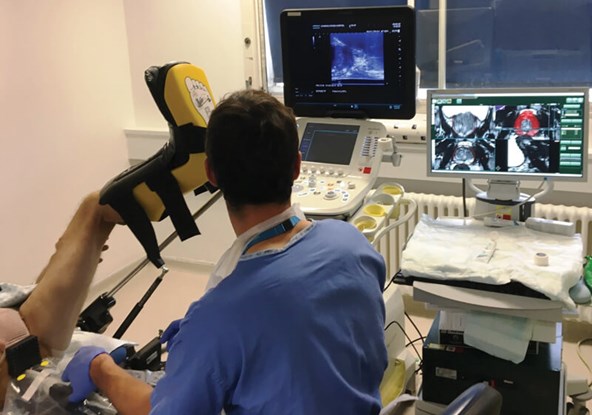
Figure 1: Image-fusion targeted transperineal prostate biopsy
(Hitachi Ultrasound and MedCom BiopSee fusion system).
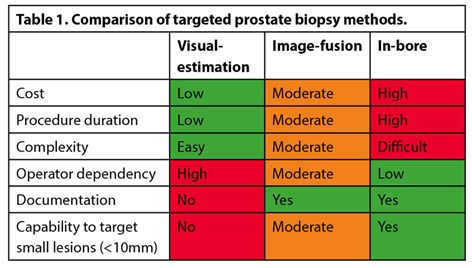
Research focus has now shifted to evaluate the clinical utility of various mpMRI targeted biopsy approaches. The three commonly used techniques are visual-estimation (cognitive), image-fusion (software) and in-bore (in-gantry) biopsy. Visual-estimation registration relies on the operator’s skill in interpreting mpMR imaging prior to biopsy and then manually targeting the lesion using TRUS guidance. Image-fusion (software) registration utilises a computer platform to overlay the contoured mpMRI lesion onto real-time TRUS imaging in either a rigid or elastic (deformable) manner, thereby guiding the operator’s biopsy needle (Figure 1). Platforms are expensive, can malfunction, and use may lengthen procedural duration. However, their use standardises technique, and allows biopsy information to be electronically recorded and reviewed at a later date should there be diagnostic doubt. In-bore biopsy (performed within the MRI scanner) involves fusion of the diagnostic mpMRI with real-time MR imaging to ensure accurate needle deployment. Although this is resource heavy and time-consuming, in-bore might offer greater accuracy (Table 1).
Whether any single targeting route yields superior cancer detection above the others remains contentious, and there are multiple aspects to consider. In this article, we contextualise current practice, discuss the available evidence in image-guided targeted prostate biopsy approaches and evaluate potential differences dependant on patient, prostate and procedural factors.
The evolution of MRI-guided prostate biopsy
Prior to evaluating MRI targeted biopsy approaches, it is useful to briefly consider its origin. Magnetic resonance imaging of the prostate was first introduced in the 1980s. This major advance allowed the substructure of the prostate (peripheral and central zones) to be reliably visualised on T2 weighted images. Unfortunately, images were limited in spatial resolution (due to larger fields of view and thicker slices) and so therapeutic application was limited [5]. It was not until the early 2000s that MRI was utilised to guide prostate biopsy. Cormack and colleagues described an in-bore biopsy of a 74-year-old patient who had previously undergone a proctocolectomy – despite having a raised PSA, four separate transurethral prostate biopsies were all negative. In a 0.5T MR scanner, eight transperineal samples were taken, two of which yielded cancer [6]. However, widespread uptake of in-bore biopsy was limited by patient discomfort, long duration, cost, and lack of specialist equipment and skillset. When prostate cancer was suspected, ‘blind’ TRUS prostate biopsies remained the standard.
Subsequent advances in MR imaging and interpretation have galvanised its use in the prostate cancer diagnostic pathway. First developed by consensus in the late 2000s, the Prostate Imaging Reporting And Data System (PI-RADS) aims to standardise prostate MRI acquisition and reporting. By combining various imaging findings, PI-RADS risk stratifies mpMRI lesions from 1 (very low risk) to 5 (very high risk). Meta-analysis yields pooled csPCa detection rates for PI-RADS version 2 of 4% for score 1-2, 17% for score 3, 46% for score 4 and 75% for score 5 [7].
The PROMIS study heralded a sea-change in biopsy approach. 576 men underwent mpMRI (as an index test) followed by both standard 10-12 core TRUS biopsies and transperineal template mapping biopsies. mpMRI sensitivity and negative predictive value was 93% and 89%, respectively; in this cohort, if mpMRI had been used as a triage tool, 27% could have safely avoided biopsy [8]. Building on this, the PRECISION study randomised men to either 10-12 core TRUS biopsy, or mpMRI with or without targeted prostate biopsy (using either visual-estimation or image-fusion depending on local expertise). MRI-targeted biopsy resulted in detection of more csPCa (38% vs. 26%, p=0.005) and less clinically insignificant (9% vs. 22%, p<0.001) cancer [9]. As the superiority of the mpMRI directed diagnostic pathway became apparent, image-fusion software platforms (which track movement of the probe to fuse TRUS and MR imaging) were developed. Initial models used electromagnetic trackers to detect the TRUS probe’s position. Subsequent iterations used a mechanical arm, and then spatially combined imaging only; biopsy accuracy and cancer detection rates of image-fusion platforms have gradually improved [10].
Is greater accuracy really needed?
Comparisons of radiological and histopathological lesion volumes have shown that mpMRI underestimates the size and extent of malignant lesions. Priester et al. examined mpMRI and whole-mount pathology specimens of 114 men who underwent radical prostatectomy. The mean tumour volume was three-times greater than the corresponding mpMRI region of interest (p=0.01) [11]. In a similar study of 37 men, Le Nobin et al. report volume underestimations of 32% and 47% on T2 weighted imaging and apparent diffusion coefficient respectively compared to histology [12]. As prostate cancer lesions lie on a spectrum from dense malignancy to a few abnormal cells in normal tissue, it can be very hard to differentiate the latter on mpMRI [13]. Consequently, mpMRI lesions may have surrounding ‘MRI invisible’ disease. This means that, even if the biopsy needle does not quite hit the target, it may still detect the cancer.
Current evidence: comparative image-guided targeted prostate biopsy trials
So, what does the comparative evidence show? Unfortunately, there is a lot of heterogeneity in the design of studies to date. Baseline populations include biopsy naïve men, those with prior negative biopsy, or both. Men may have been biopsied using visual-estimation, image-fusion, or in-bore; systematic biopsy may or may not have been used as the standard. Biopsy routes differ – transrectal might be compared to transperineal outcomes. A wide variety of image-fusion platforms utilising rigid or elastic registration have been used. Individual operator expertise is rarely reported. The number of cores taken may vary, as might the anaesthetic route. To further complicate the presentation of results, multiple definitions of what constitutes csPCa have been used. Finally, analyses have been conducted and results reported on patient and lesion-based levels.
Without discussion of such variations in protocols it is, perhaps, unsurprising that the majority of studies report no significant differences in overall cancer or csPCa (subsequently defined as any Gleason ≥3+4 unless otherwise stated) detection rates between visual-estimation, image-fusion and in-bore biopsy. Comparisons of pooled estimates from a systematic meta-analysis suggested that no one technique is superior in detecting clinically significant cancer [14]. This is supported by the subsequent FUTURE trial, which randomised 655 men with suspicion of prostate cancer to visual-estimation, image-fusion or in-bore targeted biopsy. There were no significant differences in cancer detection rates between techniques not only overall but also on various subgroup comparisons (PI‑RADS score, lesion location and prostate volume) [15]. However, the literature is not entirely unanimous, and there are some hints that techniques are not completely equivocal.
Visual-estimation versus image-fusion
Lesion location and size may matter. In the PROFUS study, 125 men underwent two transrectal targeted cores. A blinded second operator then took two visually-estimated targeted cores before further systematic biopsies. Although there were no differences in overall cancer or csPCa detection rates multivariate analysis of positive image-fusion and negative visual-estimation targeted biopsies noted that image-fusion had a significantly higher detection rate in anterior lesions (OR 3.84, p=0.05) and those of smaller diameter (OR 0.83, p=0.005) [16]. The converse was reported in a study of 200 men undergoing repeat biopsy; transperineal visual-estimation missed less csPCa (Gleason ≥3+4 and/or >2 positive cores) in the anterior zone compared to transrectal image-fusion biopsy (1 vs. 12, p=0.001) [17]. A study of 396 regions-of-interest in 286 men undergoing transrectal visual-estimation and image-fusion biopsies again found no overall difference in csPCa detection. However, image-fusion found significantly more cancer in the transition zone (p=0.046) [18].
What about when comparisons are made by mpMRI PI-RADS score? Kam et al. indirectly compared men undergoing either transrectal visual-estimation or transperineal image-fusion registration biopsies with systematic cores as the standard. Of 121 men included, there was no difference in csPCa detection rate (p=0.084). However, when considering just those with mpMRI lesions scored PI-RADS 4 or 5, visual-estimation biopsy was superior (91% vs. 71%, p=0.03) [19]. The opposite was seen by Oderda et al. in their study of 50 men – in those with PI-RADS 4 target lesions, image-fusion provided superior overall cancer detection (16.7% vs. 57.9%, p=0.05) [20].
Urologists conventionally use ‘centroid’ targeting (i.e. aiming towards the centre of the lesion). However, simulation evidence suggests that image-fusion ‘ring’ targeting may provide a superior cancer yield compared to visual-estimated ‘centroid’ targeting, as it mitigates for guidance system, image registration and random errors, although this has not been evaluated in a trial setting (Figure 2) [21].
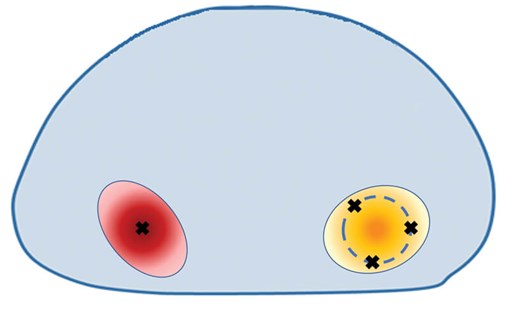
Figure 2: Centroid (red lesion) vs. ring (orange lesion) targeting.
Whether there are any true differences between visual-estimation and image-fusion by other factors remains to be seen. For example, image-fusion registration is known to have a long learning curve [22‑24], so does operator experience play a role? What if there is more than one mpMRI target lesion? Is there a difference by anaesthetic route (local vs. sedation vs. general)? Preliminary analysis of the Rapid Assessment and Prostate Imaging for Diagnosis (RAPID) registry suggests image-fusion may well be advantageous in certain subgroups [25].
Visual-estimation versus in-bore
Osses et al. compared 64 men biopsied using visual-estimation to 155 who underwent in-bore biopsies. csPCa detection rates were similar, and no differences were seen based on PI-RADS score or lesion area. However, in lesions <1.5ml, in-bore was more accurate (39% (9/23) vs. 69% (69/92), p=0.009) [26]. A retrospective Australian study comparing biopsy outcomes in 482 patients with 595 mpMRI target lesions had similar findings. Two hundred and ninety-eight lesions were biopsied in-bore, with the remainder biopsied using visual estimation (either transperineal or transrectal). Again, there were no differences in csPCa (>4 mm Gleason ≥3+4 or >6 mm Gleason ≥3+3) detection overall, by PI-RADS score or by anatomical area. In this study, there was no difference by lesion volume [27]. Finally, Zhang et al. compared 85 men biopsied using visual estimation with 88 undergoing in-bore biopsy. Although in-bore had a superior overall cancer detection rate (36.5% vs. 52.3%, p=0.037), there was no difference in csPCa detection (23.5% vs. 29.5%, p=0.371); in-bore detected more insignificant cancers [28].
Image-fusion versus in-bore
Studies comparing image-fusion and in-bore biopsy are sparse. One RCT allocated 110 men to image-fusion plus systematic 12-core TRUS biopsy and 104 to in-bore. There was no difference in overall cancer or csPCa detection [29]. Similarly, Venderink et al. retrospectively compared 278 men undergoing image-fusion or in-bore biopsy, with no difference in overall csPCa detection noted. However, although in-bore biopsy showed clinically relevant improved detection at all lesion sizes, this was greatest in smaller lesions (8mm: 49% vs 61.2%) [30].
What might the future hold?
Over the last 20 years, the importance of MRI in prostate cancer diagnosis has been established. But will other imaging modalities come to the fore? Radiotracers targeting prostate specific membrane antigen (PSMA) have increasingly been used for early detection of recurrent prostate cancer but may yet have a role in initial diagnosis [31]. Donato et al. evaluated concordance of preoperative PSMA PET/CT and mpMRI with biopsy histopathology and radical prostatectomy specimens (when available) in 144 men.
Although both imaging modalities had high rates of csPCa detection, PSMA PET/CT was superior in detecting secondary cancer foci and smaller lesions [32]. In 31 men with persistently elevated prostate specific antigen but previous negative biopsies, PSMA PET-ultrasound targeted image-fusion biopsy yielded clinically significant disease in 12 (38.7%) [33]. As radiotracers become more readily available and use less restricted, PSMA PET/CT, or PET/MRI to mitigate radiation dose, may be used alongside or even instead of mpMRI in identifying prostate biopsy targets.
Will there come a point at which the reporting radiologist or the biopsying urologist is rendered superfluous? Recent years have seen machine learning techniques applied to prostate diagnostics. Rather than rigidly matching outputs to inputs, machine learning utilises statistical tools to enable improvement in output through experience; the algorithm can change [34]. This technique has been applied to prostate cancer diagnosis. For example, on prostate mpMRI, there are multiple radiomic features unappreciable by the naked eye that may indicate malignancy [35]. Several high accuracy machine learning diagnostic algorithms exist. Litjens et al. developed a machine learning algorithm which can segment and interpret prostate mpMRI. When evaluated in 347 consecutive patients using MR-guided biopsy as the reference standard, area under the curve was 0.889 [36]. Machine learning has also been used to increase accuracy of real-time deformable registration of mpMRI and TRUS imaging, with excellent concordance with human expertly labelled imaging [37]. One could foresee such a time when such advances are implemented in an image-fusion or in-bore robotic device to automate prostate biopsy [38,39].
Conclusion
“The machine is only a tool after all, which can help humanity progress faster by taking some of the burdens of calculations and interpretations off its back” – Isaac Asimov, The Evitable Conflict.
At present, the available evidence suggests equivalence between visual-estimation registration, image-fusion registration and in-bore targeting. However, newer iterations of image-fusion platforms may offer the greatest balance of accuracy, cost-effectiveness and ease of use. Visual-estimation may be the most straightforward choice for larger, diffuse lesions, with in-bore used in selected cases for men with ongoing suspicion of cancer despite negative visual-estimation or image-fusion biopsy. Urologists have traditionally been quick to embrace new technologies and if image-fusion platforms providing diagnostic advantages in certain situations (dependant on anatomical, mpMRI and operator factors) are developed, to offer the best to our patients, we are likely to embrace such change.
References
1. Drost FJH, Osses DF, Nieboer D, Steyerberg EW, et al. Prostate MRI, with or without MRI‐targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev 2019;4(4):CD012663.
2. Lomas DJ, Ahmed HU. All change in the prostate cancer diagnostic pathway. Nature Reviews Clinical Oncology 2020;17(6):372-81.
3. De Santis M, Fanti S, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. 2019.
4. National Institute for Health & Care Excellence. NICE guideline NG131: Prostate Cancer: Biology, Diagnosis and Management. 2019
[https://www.nice.org.uk/guidance/ng131].
5. Tempany C, Straus S, Hata N, Haker S. MR-Guided Prostate Interventions. J Magn Reson Imaging 2008;27(2):356–67.
6. Cormack RA, D’Amico AV, Hata N, et al. Feasibility of transperineal prostate biopsy under interventional magnetic resonance guidance. Urology 2000;56(4):663-4.
7. Park KJ, Choi SH, Lee JS, et al. Risk stratification of prostate cancer according to pi-rads® version 2 categories: meta-analysis for prospective studies. J Urol 2020;204(6):1141–9.
8. Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet [Internet] 2017;389(10071):815–22.
9. Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378(19):1767–77.
10. Pandian SK, Challacombe B, Hammadeh M, et al. History of prostate biopsy - part 2. Urol News 2018;22(6):19-22.
11. Priester A, Natarajan S, Khoshnoodi P, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol 2017;197(2):320-6.
12. Le Nobin J, Orczyk C, Deng FM, et al. Prostate tumour volumes: Evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int 2014;114(6):E105–12.
13. Sathianathen NJ, Christidis D, Konety BR, Lawrentschuk NL. Magnetic resonance imaging cognitive fusion biopsy – is near enough good enough? BJU Int 2018;121(3):324–6.
14. Wegelin O, van Melick HHE, Hooft L, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol 2017;71(4):517–31.
15. Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur Urol 2019;75(4):582–90.
16. Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: The PROFUS trial. Eur Urol 2014;66(2):343–51.
17. Pepe P, Garufi A, Priolo G, Pennisi M. Transperineal Versus Transrectal MRI/TRUS Fusion Targeted Biopsy: Detection Rate of Clinically Significant Prostate Cancer. Clin Genitourin Cancer 2017;15(1):e33-e36.
18. Lee DJ, Recabal P, Sjoberg DD, et al. Comparative Effectiveness of Targeted Prostate Biopsy Using Magnetic Resonance Imaging Ultrasound Fusion Software and Visual Targeting: a Prospective Study. J Urol 2016;196(3):697–702.
19. Kam J, Yuminaga Y, Kim R, et al. Does magnetic resonance imaging–guided biopsy improve prostate cancer detection? A comparison of systematic, cognitive fusion and ultrasound fusion prostate biopsy. Prostate Int 2018;6(3):88–93.
20. Oderda M, Battisti G. Prostate Cancer Detection Rate with Koelis Fusion Biopsies versus Cognitive Biopsies: A Comparative Study. Urol Int 2016;97(2):230-7.
21. Martin PR, Cool DW, Fenster A, Ward AD. A comparison of prostate tumor targeting strategies using magnetic resonance imaging-Targeted, transrectal ultrasound-guided fusion biopsy: Med Phys 2018;45(3):1018–28.
22. Gaziev G, Wadhwa K, Barrett T, et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int 2016;117(1):80–6.
23. Meng X, Rosenkrantz AB, Huang R, et al. The Institutional Learning Curve of Magnetic Resonance Imaging-Ultrasound Fusion Targeted Prostate Biopsy: Temporal Improvements in Cancer Detection in 4 Years. J Urol 2018;200(5):1022–9.
24. Calio B, Sidana A, Sugano D, et al. Changes in prostate cancer detection rate of MRI-TRUS fusion vs systematic biopsy over time: Evidence of a learning curve. Prostate Cancer Prostatic Dis 2017;20(4):436–41.
25. Khoo CC, Eldred-Evans D, Peters M, et al. MP56-02 Man vs. Machine: Comparative Effectiveness of Cognitive Targeted and Image-Fusion Targeted Transperineal Prostate Biopsy. J Urol 2020;203:e847–8.
26. Osses DF, Van Asten JJ, Tijsterman JD. Cognitive-targeted versus magnetic resonance imaging-guided prostate biopsy in prostate cancer detection. Curr Urol 2018;11(4):182–8.
27. Yaxley AJ, Yaxley JW, Pokorny MR, et al. Comparison between target magnetic resonance imaging (MRI) in-gantry and cognitively directed transperineal or transrectal-guided prostate biopsies for Prostate Imaging – Reporting and Data System ( PI-RADS ) 3 – 5 MRI lesions. BJU Int 2017;120 Suppl 3:43-50.
28. Zhang K, Zhang Z, Liu M, et al. Comparison of clinically significant prostate cancer detection by MRI cognitive biopsy and in-bore MRI-targeted biopsy for naïve biopsy patients. Transl Androl Urol 2020;9(2):243-9.
29. Arsov C, Rabenalt R, Blondin D, et al. Prospective Randomized Trial Comparing Magnetic Resonance Imaging (MRI) -guided In-bore Biopsy to MRI-ultrasound Fusion and Transrectal Ultrasound-guided Prostate Biopsy in Patients with Prior Negative Biopsies. Eur Urol 2015;68(4):713-20.
30. Venderink W, van der Leest M, van Luijtelaar A, et al. Retrospective comparison of direct in-bore magnetic resonance imaging (MRI)-guided biopsy and fusion-guided biopsy in patients with MRI lesions which are likely or highly likely to be clinically significant prostate cancer. World J Urol 2017;35(12):1849–55.
31. Meyer AR, Joice GA, Allaf ME, et al. Integration of PSMA-targeted PET imaging into the armamentarium for detecting clinically significant prostate cancer. Curr Opin Urol 2018;28(6):493–8.
32. Donato P, Morton A, Yaxley J, et al. 68Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET/CT guided biopsy the future? Eur J Nucl Med Mol Imaging 2020;47(8):1843–51.
33. Liu C, Liu T, Zhang Z, et al. PSMA PET/CT and standard plus PET/CT-Ultrasound fusion targeted prostate biopsy can diagnose clinically significant prostate cancer in men with previous negative biopsies. J Nucl Med 2020;
[https://jnm.snmjournals.org/content/
early/2020/02/06/jnumed.119.235333].
34. Goldenberg SL, Nir G, Salcudean SE. A new era: artificial intelligence and machine learning in prostate cancer. Nat Rev Urol 2019;16(7):391–403.
35. Sun Y, Reynolds HM, Parameswaran B, Multiparametric MRI and radiomics in prostate cancer: a review. Australas Phys Eng Sci Med 2019;42(1):3–25.
36. Litjens G, Debats O, Barentsz J, et al. Computer-aided detection of prostate cancer in MRI. IEEE Trans Med Imaging 2014;33(5):1083–92.
37. Anas EMA, Mousavi P, Abolmaesumi P. A deep learning approach for real time prostate segmentation in freehand ultrasound guided biopsy. Med Image Anal 2018;48:107–16.
38. Miah S, Servian P, Patel A, et al. A prospective analysis of robotic targeted MRI-US fusion prostate biopsy using the centroid targeting approach. J Robot Surg 2020;14(1):69–74.
39. Barral M, Lefevre A, Camparo P, et al. In-bore transrectal MRI–guided biopsy with robotic assistance in the diagnosis of prostate cancer: An analysis of 57 patients. Am J Roentgenol 2019;213(4):W171-W179.
Declaration of competing interests: Martin J Connor receives grant funding from the Wellcome Trust and University College London Hospitals (UCLH) Charity.Hashim U Ahmed’s research is supported by core funding from the United Kingdom’s National Institute of Health Research (NIHR) Imperial Biomedical Research Centre. Ahmed currently receives funding from the Wellcome Trust, Medical Research Council (UK), Prostate Cancer UK, Cancer Research UK, The BMA Foundation, The Urology Foundation, The Imperial Health Charity, Sonacare Inc., Trod Medical and Sophiris Biocorp for trials and studies in prostate cancer. Ahmed is a paid medical consultant for Sophiris Biocorp, Sonacare Inc. and BTG/Galil; and a paid proctor for HIFU, cryotherapy and Rezum water vapour therapy.
Disclaimer: The authors did not receive any incentives (financial or otherwise) from Hitachi Medical Systems for the preparation of this article.