Part 2 of this topic is available here.
Prostate biopsy (PBx) to exclude cancer has been part of clinical practice since the beginning of the 20th Century. PBx techniques have evolved over time to optimally address some of the unique issues of this procedure, including the awkward anatomical position of the prostate, the proximity of the biopsy tract to faeces and urine, the risk of sepsis, the potential side-effects affecting voiding and sexual function, heterogeneity of the underlying cancer, discrepancy in the appearance of significant lesions between the different imaging modalities, and finally difficulty in precisely targeting the significant cancer. In addition, prostate cancer (PCa) diagnostics have always been heaped in controversy, in terms of whom to biopsy and when.
Whilst a digital rectal examination (DRE) to guide the decision regarding the need for PBx was critical in the early 1900s, the arrival of prostate specific antigen (PSA) and ultrasound (US) into clinical practice in the 1980s and the evolution of multi-parametric magnetic resonance imaging (mpMRI) in the early 21st Century have driven the surgical art of PBx into a more scientific-based procedure.
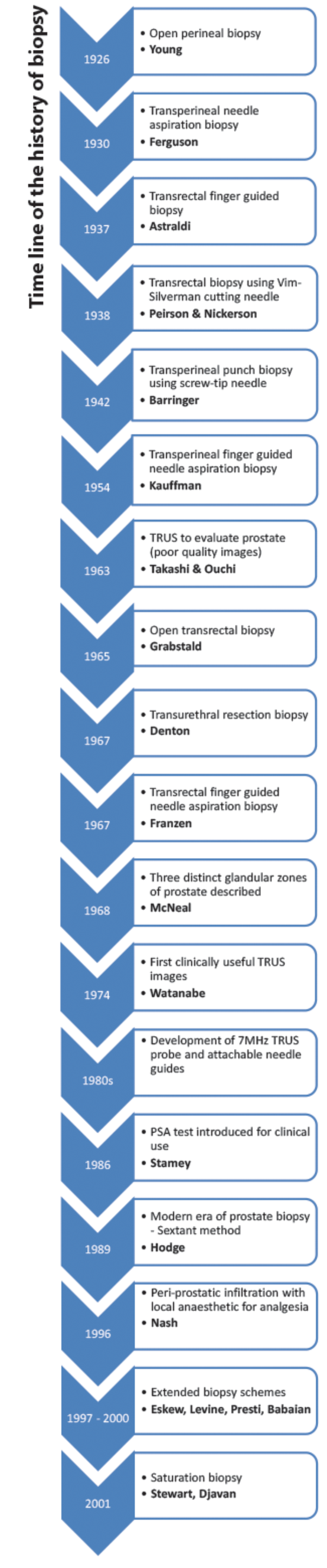
Transperineal prostate biopsy of the past
The first PBx was performed using an open perineal technique in 1926 [1]. A transverse incision was made between the two ischial tuberosities, 2cm above the anus. Dissection to reach the prostate was through the ischio-rectal fossa, when the prostate capsule was opened and tissue excised. If cancer was likely, the surgeon proceeded with a partial or a total prostatectomy. Therefore, the decision to do a PBx was not made lightly and made only when a prostatectomy was considered. Moreover, patients required general anaesthesia (GA), at least a week’s postoperative stay in hospital and almost invariably had urinary incontinence (UI) and erectile dysfunction (ED), rendering the procedure, “not a commonly used one” [1].
Open perineal PBx rightly evolved into the minimally invasive transperineal (TP) needle punch (Barringer) and needle aspiration (Ferguson) biopsy [2]. TP needle punch PBx was performed using a screw-tip needle, which allowed removal of sufficient tissue for histopathology. This technique was modified into a TP needle aspiration biopsy whereby an 18-gauge needle was inserted into the perineum away from the midline, with the patient in the lithotomy position, under local anaesthesia (LA). However, with the needle aspiration the quantity of tissue removed was poor and diagnosis of malignancy was nearly impossible.
Finger guided TP aspiration PBx was first introduced by Kaufman in 1954 [3], which involved insertion of a needle through the perineum 1cm above the anus, but guided by a digit in the rectum. Guiding allowed finer control of the needle – with the surgeon following the point along its journey within the anus to reach the nodule on the prostate. With these improvements, an overall accuracy rate of 88% was reported. Risks of ED, UI or injury to the rectum were far less, tissue sections could be permanently fixed and repeat biopsies, when needed, were simple.
Transurethral prostate biopsy
The transurethral approach required GA and a period of hospitalisation. Denton et al. were of the view that an extensive transurethral prostatectomy (TURP) would nearly always confirm the diagnosis of PCa [4]. However, Grabstald commented that TURP might be useful only in advanced tumours [5]. By then, it was also well known that PCa was more frequently seen posteriorly, near the capsule and thus was not easily reached with the resecting loop. In a series published by Peirson and Nickerson [6], one patient had only benign tissue resected for histology during transurethral PBx. However, since DRE was suspicious for cancer, a perineal punch PBx was performed and this subsequently revealed malignancy. Consequently, it was affirmed that the transurethral PBx should not be performed as a primary PBx technique but could be useful to perform TURP in those with obstructive urinary symptoms.
Transrectal prostate biopsy
Originally the transrectal (TR) approach was not preferred over the TP approach due to the worry of faecal contamination. Yet, in 1937 a technique for finger-guided TR biopsy was described for the first time [7], and it became more widely used in the 1950s, as it was considered to have the potential for increased diagnostic accuracy relative to TP biopsy. With the first digit placed into the rectum, the needle tip was placed in line with and next to the fingertip, giving good control. Some surgeons also placed a urethral sound in the bladder to push the prostate down to help reach the prostate more easily. Open TR biopsy through a proctotomy incision was also described in the 1960s by Grabstald [5], which gave good access to the posterior lobe of the prostate – the area most implicated in cancer. However, there were disadvantages; subsequent recto-urethral fistulae were reported and further radical surgery after this approach was not easy.
“By intuition, urologists began sampling more prostatic tissue through more needle cores although such procedures were perceived to cause more pain.”
Different TR needles were used to collect tissue for cytological or histological diagnosis. For example, Franzen developed a fine needle and guide for prostatic aspiration through the TR route [8]. Peirson and Nickerson were the first to use the Silverman needle, designed in 1938, to take prostatic tissue through the TR approach [6]. The Franzen needle provided only cytological diagnosis, but it had the advantages of being an outpatient procedure and had less morbidity. However, the likelihood of missing a cancer was greater compared with using the Silverman needle. The Silverman needle provided histopathological diagnosis and resulted in a lower false negative rate but required GA. In 1971 Henry and Williams concluded that the Franzen needle aspiration could be used as a first-line investigation, and should the cytology prove negative then it could be repeated again, before the urologist proceeded to use the Silverman needle biopsy [9].
Transrectal ultrasound (TRUS) guided biopsy
Development of ultrasound imaging gradually eclipsed the finger-guided prostate biopsy techniques. Takahashi and Ouchi first described the use of TRUS to evaluate the prostate in 1963 [10]. However, the image quality was too poor to be of any clinical use at that time. In 1974 Watanabe et al. were the first to demonstrate clinically useful TRUS images of the prostate [11]. They used a 3.5MHz probe, which was considered to be state of the art at the time. However, image quality continued to be unsatisfactory. It was only in the 1980s, with technological advances in probe manufacture and the development of an attachable biopsy needle guide, that TRUS became clinically useful for the diagnosis of PCa. A 7MHz probe was developed allowing better definition of the structure of the prostate.
In 1968 McNeal proposed that the prostate was composed of three distinct glandular zones, namely the transition zone (TZ), peripheral zone (PZ) and central zone (CZ) [12]. The clinical relevance of these anatomical zones became important as it was evident that the majority (70-80%) of cancers arose in the PZ. Research into TRUS appearances of PCa confirmed varying appearances especially of early stage lesions which were often indistinct from normal prostatic tissue, indicating that TRUS as a diagnostic tool lacked specificity and had limitations. With the widespread use of serum PSA testing there was an increase in the detection of early stage, low grade, low volume cancers that did not necessarily have any palpable abnormality or specific TRUS appearances. In response to this, the method of prostate sampling changed significantly in 1989.
Dawn of the modern era of prostate biopsy
The sextant method
In their first paper Hodge et al. described targeted TR PBx of palpable abnormalities, 90% of which had corresponding hypoechoic lesions on TRUS [13]. In addition, they also took biopsies of isoechoic areas in the PZ and CZ. Although these biopsies were not systematic, they were positive in 66% of cases. Their second article, published later in 1989, was a landmark paper ushering in the modern era of PBx [14]. Hodge et al. compared the TRUS-guided PBx taken from palpable or sonographic abnormalities with those taken in a random systematic fashion from six sites: the apex, middle and base of each prostate lobe. This sextant technique detected 9% more cancers compared with the targeted method. The Hodge protocol of systematic sextant biopsy of the prostate thus came to be considered the gold standard for many years in an era when a raised PSA was an acceptable indication for PBx irrespective of DRE or imaging findings.
Extended biopsy schemes
By intuition, urologists began sampling more prostatic tissue through more needle cores although such procedures were perceived to cause more pain. The reported discomfort rates were 65-90% and this discomfort was proportional to the number of cores taken [15]. In 1996 Nash et al. reported that effective pain relief could be achieved by infiltrating local anaesthesia in the peri-prostatic area for nerve blockade enabling them to take 10 to 20 biopsies [16].
Extended protocols included the five-region biopsy protocol [17], the two independent consecutive sets of sextant biopsies in the same sitting protocol [18], 10-core protocol with two extra biopsies laterally on each side at the base and mid gland [19], and an 11-core biopsy protocol including the anterior horn and TZ on each side in addition to one mid-gland biopsy [20]. Using these additional zones led to an up to 33% increase in PCa diagnosis. Studies carried out on digitally-reconstructed radical prostatectomy (RP) specimens also concluded that a 10- or 12-core, laterally-directed biopsy protocol would detect 99% of the cancers, while the standard sextant protocol would detect only 72.6% [21].
One prospective randomised study assessed the pain and morbidity associated with six biopsies compared with 12 and reported that there was no difference in the discomfort experienced, and no increase in moderate or major problems, although there was a higher rate of haematospermia (89% versus 71%) and rectal bleeding (24% versus 10%) when 12 biopsies were taken [22].
Saturation TRUS biopsies
The term ‘saturation biopsy’ was coined by Stewart et al. in 2001 [23], in which 20 or more systematic cores were taken. These saturation biopsies have been offered to those who have had previous negative biopsies but continue to raise clinical suspicion for PCa usually through a rising PSA test. This technique is generally not considered as an initial biopsy strategy as the cancer detection rate compared with the extended biopsy protocols is no higher. Djavan et al. published a series in 2001 where a 24-core biopsy template was used in 116 patients with a previous negative biopsy and yet suspicious findings for a missed tumour and demonstrated a 41% cancer detection rate [24].
The major limitations of these sextant and extended TRUS PBx protocols were the persistently significant false negative rates and the anterior prostate especially being under-sampled. To address these limitations, the transperineal PBx has once again resurfaced in clinical practice ushering in another era of prostate biopsy.
For Part 2 of this topic please click here.
References
1. Young HH, Davis DM. Young’s practice of urology: based on a study of 12,500 cases. London, UK; WB Saunders; 1926.
2. Barringer B. Prostatic carcinoma. J Urol 1942;47:306-10.
3. Kaufman JJ, Rosenthal M, Goodwin WE. Needle biopsy in diagnosis of prostatic cancer. California Medicine 1954;81(5):308-13.
4. Denton SE, Valk WL, Jacobson JM, et al. Comparison of the perineal needle biopsy and the transurethral prostatectomy in the diagnosis of prostatic carcinoma: an analysis of 300 cases. J Urol 1967;97:127.
5. Grabstald H. Biopsy techniques in the diagnosis of cancer of the prostate. CA: A Cancer Journal for Clinicians 1965;15:134-7.
6. Peirson EL, Nickerson DA. Biopsy of the prostate with the Silverman needle. New England Journal of Medicine 1943;228(21):675-8.
7. Astraldi A. Diagnosis of cancer of the prostate: biopsy by rectal route. Urol Cutaneous Rev 1937;41:421-2.
8. Williams JP, Still BM, Pugh RCB. The diagnosis of prostatic cancer: cytological and biochemical studies using the franzen biopsy needle. British Journal of Urology 1967;39:549.
9. Hendry WF, Williams JP. Transrectal prostatic biopsy. BMJ 1971;4:595-7.
10. Takahashi H, Ouchi T. The ultrasonic diagnosis in the field of urology. Proc Jpn Soc Ultrasonics Med 1963;3:7.
11. Watanabe H, Igari D, Tanahasi Y, et al. Development and application of new equipment for transrectal ultrasonography. J Clin Ultrasound 1974;2:91-8.
12. McNeal JE. Regional morphology and pathology of the prostate. Am J Clin Pathol 1968;49:347.
13. Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol 1989;142:66-7.
14. Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol 1989;142:71-4.
15. Stamey TA. Making the most out of six systematic sextant biopsies. Urology 1995;45:2-12.
16. Nash PA, Bruce JE, Indudhara R, et al. Transrectal ultrasound guided prostatic nerve blockade eases systematic needle biopsy of the prostate. J Urol 1996;155:607-9.
17. Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol 1997;157:199-202.
18. Levine MA, Ittman M, Melamed J, et al. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol 1998;159:471-5.
19. Presti JC, Chang JJ, Bhargava V, et al. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol 2000;163:163-6.
20. Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy Strategy. J Urol 2000;163:152-7.
21. Bauer JJ, Zeng J, Weir J, et al. Three-dimensional computer-simulated prostate models: Lateral prostate biopsies increase the detection rate of prostate cancer. Urology 1999;53:961-7.
22. Naughton CK, Ornstein DK, Smith DS, et al. Pain and morbidity of transrectal ultrasound guided prostate biopsy: A prospective randomized trial of 6 versus 12 cores. J Urol 2000;163:168-71.
23. Stewart CS, Leibovich BC, Weaver AL, et al. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol 2001;166(1):86-91.
24. Djavan B, Ravery V, Ziotta AR. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol 2001;166:1269-83.
Declaration of competing interests: None declared.