When less is more: percutaneous biopsy and tumour seeding in papillary renal cell carcinoma
Renal cell carcinoma (RCC) accounted for 2.2% of new cancer diagnoses worldwide in 2018 with over 400,000 new cases and 175,098 deaths [1]. The majority of RCCs are classified as clear cell (70%) followed by papillary RCC and chromophobe RCC [2]. Early-stage renal cell carcinoma has a good five-year survival rate, around 93%, however this figure significantly drops for those patients presenting with metastatic RCC with a five-year survival rate of 12% [3].
Percutaneous biopsy of renal masses is an effective investigation to distinguish between benign and malignant renal masses with a low complication rate (<5%) and a high diagnostic accuracy of over 90% [4]. It is also used to guide systemic therapy and specific treatments such as immune checkpoint inhibitors in patients with advanced or metastatic disease, and as a decision aide in small or atypical lesions for which active surveillance or ablative therapy has been suggested [5], particularly if the patient may not be a good candidate for surgery.
Tumour seeding following biopsy of a renal mass has been reported previously with various estimations of incidence, both generally and when broken down into histology subtypes. The following report will focus on one of our local cases of seeding following biopsy of a papillary RCC.
Case
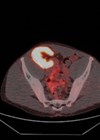
Our patient is a 42-year-old man who was referred to the department after a CT scan found two left sided renal masses suspicious for papillary RCC, an interaortacaval lymph node mass that appeared to be a separate pathology (later found to be a paraganglioma) and multiple bone lesions in his iliac joints and right seventh rib (Figure 1).
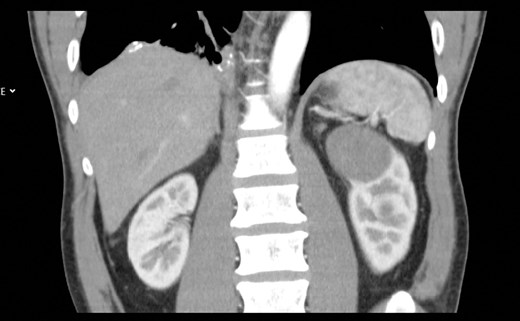
Figure 1: Presenting CT arterial portography of the patient, post contrast coronal,
note the bland looking homogeneous tumour in the upper pole of the left kidney.
He was asymptomatic with regards to the renal masses and had originally presented to hospital with headaches. The decision was made to do a left sided percutaneous core biopsy under CT guidance. During the procedure there were no intraoperative concerns regarding tumour manipulation or bursting. The histopathology returned as likely papillary RCC, no normal renal parenchyma seen. Two months later the patient underwent a laparotomy in which the interaortacaval paraganglioma was excised at the same time as a left open partial nephrectomy of the larger mass. Postoperative histology of the renal mass revealed a 60mm type 1 papillary RCC with tumour seen within the peri-nephric fat (T3a). A subsequent re-review of the renal core biopsy by our histopathologists found an area of tumour rupture within the perinephric fat, beyond which was a small focus of tumour lying within a linear band of fibrous tissue (Figure 2). The histopathologists regarded this as perinephric fat involvement by the tumour either due to tumour cell spillage as a consequence of tumour rupture, or more likely by tumour seeding along the needle tract of the previous core biopsy (supported by the linear band of tissue seen around the tumour focus). As a result the final tumour staging was recorded as pT3a.
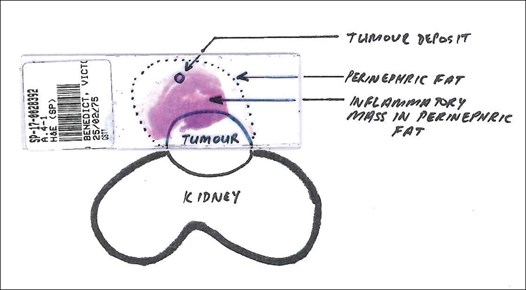
Figure 2: Histology slide from initial renal mass biopsy showing tumour deposits in the perinephric fat.
Our patient was closely followed up after discharge, as the smaller of the two renal masses had not been removed. One year later he underwent a redo left open partial nephrectomy as this mass had grown in size, now measuring 35mm. Histology of this mass reported type 1 papillary RCC, pT1a. Following this the patient continues to be well, and three years later there are no clinical, radiological or biochemical signs of recurrence of either renal cell carcinoma or paraganglioma.
Discussion
Our case is an example of a tumour being upstaged as a result of external factors rather than intrinsic tumour behaviour. Tumour seeding is defined as the process in which tumour cells are deposited along the tract made by a biopsy needle, and has long been listed as a risk of percutaneous biopsy in both renal and other types of tumours. It is made possible by tumour cells having lower cell-to-cell adhesion than normal tissue, making them easier to dislodge [6]. Plus tumour cells usually have excellent vascularity allowing dislodged tumour cells to disseminate further afield via the bloodstream or lymphatic system, particularly when blood vessels have been damaged by a biopsy needle, resulting in distant metastases. This can have fatal consequences. On the other hand, in some cases tumour seeding appears to have little significant effect other than on the technical tumour staging. Our case study is one of many examples of local spread of cancer following diagnostic biopsy. Significantly, it highlights the particular risk of subcutaneous biopsy on masses that are suspicious for papillary RCC.
There are two things to consider when weighing up the risk of seeding; both how common an occurrence it is and the clinical impact confirmed seeding has on the patient. Needle tract tumour seeding to perinephric tissue has been observed in the literature previously, but with various estimations in the incidence. For example, in 1995 it was estimated to be as low as 0.01% [7], but a case series in 2019 reported an incidence of 1.2% [8]. It is worth noting that in this 2019 case series six out of the seven cases reported were papillary histology, and the remaining one was clear cell. A larger study reviewed the National Cancer Database over a three-year period and identified over 24,000 patients who underwent surgery for clinical T1a tumours [9]. They found that there was an increased rate of upstaging to pT3a perinephric fat involvement in the group that had undergone biopsy than the group that had not (2.1% compared to 1.1%). They concluded that the effect was small and clinical significance unclear, and perhaps this effect was balanced by the significance of the information ascertaining by biopsy. It is important to note that this study found a higher rate in upstaging to perinephric fat in patients with papillary RCC compared to clear cell; 1.97% of their patients with papillary histology were upstaged compared to 0.97%.
The question here is whether tumour seeding due to a biopsy has any impact on progression-free survival or cancer specific mortality. We also need to balance this against the risk of performing surgery for a benign tumour in the case of a small renal mass. A higher frequency of seeding after biopsy was found by a longer-term study reviewing the histology of RCC nephrectomies with previous biopsy over 17 years [10]. They recorded seeding to perinephric tissue in 6% of their RCC cases, a much higher frequency than in other studies. Interestingly, none of these cases had recurrence or metastases within that timeframe. It could be argued that upstaging is not justified if there is solely seeding found along the needle tract; indeed, there is currently not a standard protocol amongst pathologists with regards to upstaging these specimens [10]. However, there are other case studies reporting multiple tumour recurrences in the years following nephrectomy with prior biopsy, in locations including the biopsy canal, abdominal wall and psoas muscle [11]. Rarely this can include miliary abdominal dissemination. Not only does this increase the burden of management and number of surgeries the patient is subjected to, but it causes the patient great emotional distress.
As previously mentioned, the ultimate histology of the tumour can impact the likelihood of tumour seeding following biopsy. In our case papillary RCC was suspected prior to biopsy due to the non-enhancing, homogenous appearance of the tumours on CT imaging, and was then confirmed by histology. Papillary tumours are softer and more delicate, often containing viscous toothpaste like material, and thus more likely to rupture and / or cause seeding following biopsy. A possible explanation is that papillary tumours are less likely to have complete peritumoural pseudocapsules compared to clear cell, helping facilitate tumour invasion to perinephric fat [12]. Papillary tumours have a significantly higher rate of invasion to parenchyma when compared to clear cell (30% compared to 8%) [12]. It is therefore sensible to caution against the use of biopsy when papillary tumours are suspected, as there is a significantly greater risk of tumour seeding.
There have been developments in the world of interventional radiology to minimise the risk of tumour seeding, namely the use of coaxial sheath needles. These are larger outer sheaths through the lumen of which smaller needles can pass through, allowing multiple biopsies to be taken from a mass with only one pass through the surrounding healthy tissue. The sample is retracted through the sheath’s lumen and so protects the parenchyma and soft tissue from direct contact with the sample. Retrospective studies have shown that in the case of hepatocellular carcinoma the use of coaxial needles have a significantly lower rate of seeding compared to non-coaxial needle biopsies (3% compared to 1.3%) [13]. Other factors that help reduce seeding risk include using the shortest needle tract possible and using negative pressure on withdrawal [14].
Conclusion
Percutaneous biopsy carries an ever-present small risk of tumour seeding, which must be weighed up against the potential benefits of gaining preoperative histology in each patient. We feel that there are many occasions in which renal mass biopsy is a wise decision, as advised by local protocols, e.g. older, frail patients, bilateral tumours and previous other malignancies. However, due to the increased risk of seeding in cases with papillary histology coupled with the usually distinct appearance of papillary tumours on imaging, we believe it is prudent to avoid biopsy in cases of suspected papillary RCC. Instead, we would advise proceeding directly to definitive treatment. If biopsy is necessary, then the perinephric fat should be excised simultaneously and sent with the specimen at the time of surgery.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394-424.
2. Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ (Clinical research ed.) 2014;349:4797.
3. Attalla K, Weng S, Voss MH, Hakimi AA. Epidemiology, risk assessment, and biomarkers for patients with advanced renal cell carcinoma. The Urologic Clinics of North America 2020;47(3):293-303.
4. Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Seminars in Interventional Radiology 2014;31(1):20-6.
5. BAUS. MDT (Multi-Disciplinary Team) guidance for managing kidney cancer. 2012
https://www.baus.org.uk/professionals/baus
_business/publications/22/renal_cancer_mdt
[accessed 2 December 2021].
6. Shyamala K, Girish HC, Murgod S. Risk of tumor cell seeding through biopsy and aspiration cytology. Journal of International Society of Preventive & Community Dentistry 2014;4(1):5.
7. Herts BR, Baker ME. The current role of percutaneous biopsy in the evaluation of renal masses. Seminars in Urologic Oncology 1995;13(4):254-61.
8. Macklin PS, Sullivan ME, Tapping CR, et al. Tumour seeding in the tract of percutaneous renal tumour biopsy: a report on seven cases from a UK tertiary referral centre. European Urology 2019;75(5):861-7.
9. Salmasi A, Faiena I, Lenis AT, et al. Association between renal mass biopsy and upstaging to perinephric fat involvement in a contemporary cohort of patients with clinical T1a renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations 2018;36(12):527.e13-e19.
10. Zhou Y, Murugan P, Li F, Bu L. Needle tract seeding in renal tumor biopsies: experience from a single institution. Diagnostic Pathology 2021;16(1):1-9.
11. Andersen MF, Norus TP. Tumor seeding with renal cell carcinoma after renal biopsy. Urology Case Reports 2016;9:43-4.
12. Jacob JM, Williamson SR, Gondim DD, et al. Characteristics of the peritumoral pseudocapsule vary predictably with histologic subtype of T1 renal neoplasms. Urology 2015;86(5):956-61.
13. Fotiadis N, De Paepe KN, Bonne L, et al. Comparison of a coaxial versus non-coaxial liver biopsy technique in an oncological setting: diagnostic yield, complications and seeding risk. European Radiology 2020;30(12):6702-8.
14. Tyagi R, Dey P. Needle tract seeding: an avoidable complication. Diagnostic Cytopathology 2014;42(7):636-40.