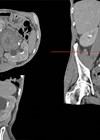
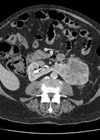
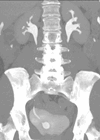
Upper tract urothelial carcinoma (UTUC) is a rare disease accounting for 5-10% of all urothelial carcinomas and has an annual incidence in Western countries of 1-2 per 100,000 [1,2]. It occurs more commonly in the pelvicalyceal system as opposed to the ureter with a 2:1 ratio. It is an aggressive disease with 60% of cases being invasive as opposed to only 20% of bladder cancers.
While radical nephroureterectomy (RNU) remains the ‘gold standard’ treatment, a substantial number of patients still succumb to their disease with five-year disease-specific mortalities of 15-30% [3-5], probably due to undiagnosed micrometastasis present at the time of diagnosis. Historically, the endoscopic management of UTUC was solely confined to imperative indications: solitary kidney, bilateral disease and end-stage chronic renal failure. However, with increasing experience endoscopic management has been extended to selected patients for elective indications – i.e. patients with low-grade tumours with normal contralateral kidneys.
Furthermore, with increasing evidence that nephron-sparing surgery may provide better overall survival in patients with renal cell carcinoma by reducing progression to chronic kidney disease (CKD), there is also interest in the outcomes of UTUC patients spared RNU by undergoing endoscopic management. In this article we discuss the evidence behind this shift in emphasis.
Background
The first endoscopic treatment of a renal pelvic tumour was performed in 1985 by the retrograde ureteroscopic approach and this was followed a few years later by the percutaneous approach [6]. Improvements in fibreoptic technology and refinements in endoscopic techniques have made endoscopic management an increasingly popular alternative. Lesions on excretory urography (that are most commonly identified as filling defects, and which are particularly suspicious if there is enhancement on the early post-contrast phase) need to be evaluated. An adequate biopsy for a histological diagnosis is essential, as previous observations have reported that a ureteroscopic visual diagnosis is only accurate in 70% of cases [7]. This is particularly important if endoscopic management, rather than nephroureterectomy, is to be offered.
The critical factors that maximise the chance of adequate histology include performing multiple biopsies using cold-cup or Piranha® (Boston Scientific) forceps and gaining good biopsy sample size. Recently, BIGopsy® back-loading biopsy forceps have been developed which provides a 4mm3 cup size providing a greater amount of biopsy material (Cook Medical). However the ureteroscope needs to be removed from the patient to present the biopsy material (as the forceps are too large to be withdrawn through the working channel of the scope). Other recognised techniques include flat wire basketing for papillary lesions and selective urine cytology including brushings. Despite the range of strategies available for obtaining biopsy material, the accuracy is still inherently limited due to small tissue volumes and crush artefact. In this regard, Bultitude et al. have reported that Bouin’s agent may be useful as an alternative fixative to formalin as it allows better preservation of nuclear architecture in small biopsy specimens [8].
Endoscopic biopsy provides accurate information regarding tumour grade and correlates well with subsequent histopathological grade at RNU in 78-91% of cases [9]. However, biopsy stage does not correlate well with histopathological stage as it cannot predict the integrity of the lamina propria and is therefore of limited value [9]. Fortunately, tumour grade appears to correlate largely with stage and can therefore be used as a guide: Charbit et al. reported that nearly 80% of grade-two or three tumours invaded into or beyond the muscularis layer [10,11]. Positive urine cytology may also be valuable in staging as it has been shown to be associated with muscle-invasive and non-organ-confined disease [12].
These factors, along with the presence of ipsilateral hydronephrosis, can be used to identify patients with high-risk features, who can then be advised that they are more likely to have a better outcome from RNU than endoscopic treatment [12]. Brien et al. has reported on 469 patients with UTUC and noted that combining ureteroscopic biopsy grade, urine cytology and hydronephrosis incrementally improved the prediction of upper tract urothelial carcinoma stage. Abnormality of all three tests (i.e. with high grade disease in the case of biopsy) gave a positive predictive value of 89% for muscle invasive disease and 73% for being non-organ confined. However, if all three tests were normal (i.e. low grade disease on biopsy), the negative predictive value for invasive or non-organ confined TCC was 100% [13].
Endoscopic treatment of UTUC
Small unifocal tumours less than 1cm in size, and which are easily accessible (i.e. within the ureter, renal pelvis, and upper calyces) are the ideal tumour for ureteroscopic management. Larger lesions may be managed more effectively through a percutaneous approach, which carries a risk of seeding via the track, therefore requiring multi-disciplinary management, including the potential to irradiate the track after the treatment has been performed. One of the commonest tools to ablate UTUC endoscopically is the use of laser and there are now a number of commercially available systems. Holmium: YAG, Thulium: YAG and Nd: YAG laser systems have all been shown to be clinically effective although there are few direct comparative trials.
Holmium: YAG laser is a pulsed energy, which emits in the infrared spectrum at 2140nM and is absorbed efficiently in water, implying good safety. Tissue penetration is less than 0.5mm making it less straightforward to use for haemostasis, but which has the advantage of minimal collateral damage. For de-bulking and clearing papillary tumours the Ho:YAG laser parameters should be set in the range 0.6-1.0J and a frequency of 5 to 10HZ. The continuous Nd:YAG laser emits energy at 1060nm with less absorption and more penetration. When set at 20-30W, it is particularly effective for tissue coagulation at a considerable depth (5-6mm), making it better suited for coagulative necrosis of large lesions, particularly in the renal pelvis.
However, Larizgoitia et al. performed a systematic review of the effectiveness of Nd:YAG laser and identified it to be less precise than other forms of laser [14]. Furthermore, it appears that the risk of subsequent ureteric stricture is higher with this form of laser due to the deep tissue penetration [15]. The other commonly used laser, Thulium: YAG operates at a wavelength of 2013nm and its effect is dependent on tissue vascularisation. Haemostasis is comparable to Ho:YAG with the advantages of a continuous wave laser beam.
“With increasing evidence that nephron-sparing surgery may provide better overall survival in patients with RCC, there is interest in the outcomes of UTUC patients spared RNU by undergoing endoscopic management.”
In discussing the key points of endoscopic management of UTUC it is important to note that at the time of writing, there is no level 1 evidence on the validity of this treatment in comparison to RNU. In fact, all studies reviewed here are either non-randomised comparative studies (level 3b) or case series (level 4). As expected, selection bias is also present in many studies with patients selected for immediate RNU more likely to have features associated with advanced disease, such as higher tumour stage, presence of lymphovascular invasion, and increased incidence of regional lymphatic disease. Elderly patients may also be more likely to undergo ureteroscopic ablation in an attempt to preserve renal function and to benefit from the lower morbidity associated with endoscopic surgery than might be expected from major surgery in this age group. In a systematic review of all English language publications up to December 2011 it was reported that the majority of tumours treated endoscopically were <2cm in size and unifocal.
Furthermore 56-66% of patients were designated as ASA 3 (American Society of Anesthesiologists Physical Status Classification System) indicating significant co-morbidities [14]. All these facts make direct oncological comparison between RNU and endoscopic surveillance difficult. The same review reported that few publications exist with long-term oncological outcomes from ureteroscopically managed UTUC with only 40 patients having outcome figures longer than 50 months [16].
One of the largest series by Cutress et al. [9] reported the results of 73 patients with a median age at diagnosis of 67.7 years. Median follow-up was 54 months, with 68% of tumours recurring. The low RFS highlights the importance of long-term follow-up with a median of four ureteroscopies performed per patient. Ultimately, 19% (n=14) of patients proceeded to RNU with a grade-dependent trend. The estimated five-year disease-specific survival (DSS) was 88.9%, with an overall survival (OS) of 69.7%, reducing to a DSS of 77.4% and OS of 40.3% at 10-years. These results point to the fact that while endoscopic management appears to provide reasonable oncological efficacy, this patient cohort is more likely to die from other causes than the disease itself.
Impact of previous endoscopic management on oncological outcome
Early publications based on case-reports raised concern over the oncological safety of ureteroscopy in UTUC [17,18]. Tomera et al. suggested that pyelovenous and pyelolymphatic backflow may occur due to the high pressure irrigation system used and suggested this caused tumour seeding, leading to recurrence and possible metastasis [16]. These fears have largely been allayed with Kulp and Bagley reporting no evidence of free or clumped tumour cells in the vascular or lymphatic system in 13 patients with UTUC who underwent RNU following ureteroscopy [19]. Hendin et al. also did not identify any significant differences in stage, grade or disease-specific survival in those patients who had been ureteroscoped [20]. The impact of delay to RNU patients in attempting endoscopic intervention is unclear, whether any deferral in definitive radical surgery increases the risk of disease progression and represents a missed window of opportunity is still to be characterised.
Boorjian et al. [21] reported that ureteroscopy with or without ablation did not appear to adversely affect postoperative disease status. This study included 208 UTUC patients of whom 121 had undergone primary RNU, 75 RNU after ureteroscopic biopsy and 12 RNU after ureteroscopic ablation. At a mean follow-up of 37 months the disease-free survival was 85%, 81% and 83%, respectively, which did not show statistical significance. However, the ablation study size was small and the mean time from ureteroscopic biopsy to RNU was 196 days making conclusions for any longer periods of time difficult. More recently, Gurbuz et al. [22] reported on a database of patients from the UTUC Collaboration (1268 patients, of whom 13% (n=175) had undergone RNU following ureteroscopic tumour ablation) and reported that the five-year DSS and disease-free survival (DFS) were 77% and 72% in those with a history of tumour ablation versus 73% and 69% without a history of ablation (p=0.171 and p=0.365, respectively). In multivariate Cox regression analysis, history of ablation therapy was found not to be associated with either disease recurrence or cancer-specific mortality.
Expanding role of endoscopic management
The newer generation of flexible ureteroscopes have a range of flexion and improved optics; this allows efficient exploration of nearly all areas of the upper urinary tract [12]. In selected patients, tumour ablation can now be performed for elective indications such as those with a normal contralateral kidney [23]. In fact the 2011 European Association of Urology (EAU) UTUC guidelines now concur that this is a viable option in suitably selected patients [12]. Thompson et al. reported on one of the largest series of 83 patients treated with endoscopic management (76 ureteroscopy and seven percutaneous) for an elective indication [23]. Mean tumour size was 0.8cm (range 0.2-3.0cm) with 10% (n=8) grade 3 tumours. Median follow-up was 4.6 years during which 55% developed upper tract recurrences, and 33% (n=27) ultimately required a nephroureterectomy.
Patients with high-grade disease (risk ratio 9.8; p=0.001) and non-Ta pathology (risk ratio 5.7; p=0.003) were at the greatest risk of death from their disease. In the elderly cohort, the five-year cancer-specific survival was deemed to be acceptable at 85%. Goel et al. reported a broadly similar conversion rate following percutaneous access with 40% ultimately undergoing RNU [24]. All patients need to be willing to conform to a strict surveillance protocol due to the high rate of recurrences.
The role of adjuvant topical chemotherapy
Due to the effectiveness of adjuvant chemotherapeutic agents in the treatment of bladder cancer a number of investigators have considered whether it has similar effect in the upper tracts. Both have a risk of toxicity if the urothelium has not had a chance to heal: the potential side-effects with upper tract Bacillus Calmette–Guérin (BCG) administration include fever, irritative voiding symptoms, flu-like illness and severe septicaemia and death; whilst, albeit very rare, agranulocytosis can occur if systemic absorption of Mitomycin C (MMC) occurs.
These agents can be delivered percutaneously or via a retrograde ureteric catheter. At present only a limited number of studies are available. Cutress et al. [9] reported on 18 patients who underwent topical MMC with an estimated five-year upper tract recurrence free survival of 53.8% versus 54.2% for those without treatment. Even when sub-stratifying for UTUC grade they were unable to show any difference. Perhaps the largest study using topical adjuvant BCG comes from Rastinehad et al. [25] who reported a 20-year experience with BCG administered percutaneously as a six-week course to 50 renal units. This group was compared with 39 controls and overall there was no statistical difference for recurrence, time to recurrence, or progression, between the treatment and control arms when stratified by grade and stage. It is reasonable to conclude that there is currently no proven efficacy for any agent in the treatment of UTUC.
Conclusion
UTUC is a relatively rare disease and, despite advances in endoscopic management, the ‘gold standard’ treatment still has to be regarded as RNU. However, this is a major operation with potential morbidity and undoubtedly results in reduced renal function (unless the removed kidney is already non-functioning). With the peak incidence of UTUC in the seventh and eighth decade of life [12], renal preservation is often an important consideration. Furthermore, many of these patients may have coexisting medical conditions, which may increase this risk of (or even preclude) radical surgery. An assessment of life expectancy must play a role particularly in the older patient who may ultimately die of non-cancer causes. With 20 years of experience in endoscopic management of UTUC there is still room to increase the amount of high-level evidence for its role. More long-term data is needed with larger patient numbers, which may require international collaboration.
Endoscopic management is a safe and effective option for low-grade disease, achieving five-year cancer-specific survival of 77-85%. While this option had previously only been considered for imperative indications it now appears that this is safe for elective indications. If positive urine cytology, unilateral hydronephrosis and high-grade disease are excluded there is good evidence that this represents low-stage disease. Clearly, patient selection is critical and patients must be counselled appropriately with respect to compliance and motivation for regular endoscopic surveillance. Recurrence-free survival is low with endoscopic treatment and the patient must commit to a programme of regular imaging and endoscopic surveillance. However, this approach appears to provide good renal preservation with only a minority (approximately 20%) progressing to RNU. Due to the low incidence of UTUC it is probably advisable that cases are managed in designated centres where resources and expertise can be concentrated.
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300.
2. Roupret M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper tract urothelial cell carcinomas: 2011 update. Eur Urol 2011;59:584-94.
3. Stewart GD, Humphries KJ, Cutress ML, et al. Long-term comparative outcomes of open versus laparoscopic nephroureterectomy for upper tract urothelial-cell carcinoma after a median follow-up of 13-years. J Endourol 2011;25:1329-35.
4. Capitanio U, Shariat SF, Isbarn H, et al. Comparison of oncological outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol 2009;56:1-9.
5. Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224-33.
6. Ritsau BT, Tomaszewski JT, Ost MC. Upper tract urothelial carcinoma: current treatment and outcomes. Urology 2012;79(4):749-56.
7. El-Hakim A, Weiss GH, Lee BR, et al. Correlation of ureteroscopic appearance with histological grade of upper tract transitional cell carcinoma. Urology 2004;63:647-50.
8. Bultitude MF, Ghani KR, Horsfield C, et al. Improving the interpretation of ureteroscopic biopsies: use of Bouin’s fixative. BJU Int 2011;108(9):1373-5.
9. Cutress ML, Stewart GD, Wells-Cole S, et al. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int 2012; [Epub ahead of print].
10. Nielson K, Ostri P. Primary tumours of the renal pelvis: evaluation of clinical and pathological features in a consecutive series of 10 years. J Urol 1988;140:19-21.
11. Charbit L, Gendreau MC, Mee S, et al. Tumours of the upper urinary tract: 10 years of experience. J Urol 1991;146:1243-6.
12. Roupret M, Zigeuner R, Palou J. European guidelines for the diagnosis and management of upper tract urothelial cell carcinomas: 2011 update. Eur Urol 2011;59:584-94.
13. Brien JC, Shariat SF, Herman MP, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 2010;184:69-73.
14. Larizgoitia I, Pons JM. A systematic review of the clinical efficacy and effectiveness of the holmium: YAG laser in urology. BJU Int 1999;84:1.
15. Bader MJ, Sroka R, Gratzke C, et al. Laser therapy for upper urinary tract transitional cell carcinoma: indications and management. Eur Urol 2009;56:65-71.
16. Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 2012; [Epub ahead of print].
17. Tomera KM, Leary FJ, Zincke H. Pyeloscopy in urothelial tumours. J Urol 1982;127:1088-9.
18. Lim DJ, Shattuck MO, Cock WA. Pyelovenous lymphatic migration of transitional cell carcinoma following flexible ureterorenoscopy. J Urol 1993;149:109-11.
19. Kulp DA, Bagley DH. Does flexible ureteropyloscopy promote local recurrence of transitional cell carcinoma? J Endourol 1994;8:111-13.
20. Hendin BH, Streem SB, Levin HS, et al. Impact of diagnostic ureteroscopy on long-term survival in patients with upper tract transitional cell carcinoma. J Urol 1999;161:783-5.
21. Boorjian S, Ng C, Munver R, et al. Impact of delay to nephroureterectomy for patients undergoing ureteroscopic biopsy and laser tumour ablation of upper tract transitional cell carcinoma. Urology 2005;66(2):283-7.
22. Gurbuz C, Youssef RF, Sharait SF, et al. The impact of previous ureteroscopic tumour ablation on oncological outcomes after radical nephroureterectomy for upper urinary tract urothelial carcinoma. J Endourol 2011;25(5):775-9.
23. Thompson RH, Krambeck AE, Lohse CM, et al. Endoscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. Urology 2008;71(4):713-17.
24. Goel MC, Mahendra V, Roberts JG. Percutaneous management of renal pelvic urothelial tumours: long-term follow up. J Urol 2003;169:925-9.
25. Rastinehad AR, Ost MC, Vanderbrink BA, et al. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncological benefit with adjuvant Bacillus Calmette Guerin therapy? Urology 2009;73:27-31.