Upper tract uroepithelial carcinoma (UTUC) is a fairly common disease which traditionally had poorer outcomes compared to bladder cancer. This is due to various factors leading to delayed diagnosis and problems in risk stratification. Continuing efforts have focused on early diagnosis and risk stratification and recently the focus has shifted to renal preservation in appropriately selected patients which will improve patient outcomes long-term that are associated with renal dysfunction [1].
Advances are being made in endourological management, and novel topical treatments offer a viable option to selected low-risk patients. Accurate risk stratification and appropriate patient selection is the key for successful endoscopic management. It is well documented that under-staging or inaccurate risk stratification leads to recurrences and poorer outcomes [2].
In this article, we explore the current challenges in both the diagnosis and management of upper tract transitional carcinomas to highlight the recent advances.
Background
Epidemiology
UTUC makes up 5% of all urothelial malignancies and 10% of renal tumours. The exact scale of the problem is difficult to quantify as they have been traditionally grouped under renal cancer. However, it is estimated to be in the region of 2 per 100,000. UTUC is more common in the renal pelvis, accounting for twice the number of cases as compared to the ureter [2]. Synchronous bladder cancer can be present in up to 17% of cases, while following radical nephroureterectomy (RNU) bladder recurrence occurs in about 22-47%. Concomitant carcinoma in situ (CIS) is present in about 11% [2]. With a primary diagnosis of bladder TCC, only 3% develop UTUC subsequently while 2-6% can develop contralateral UTUC after RNU [3].
Risk factors
Risk factors for UTUC are the same as that of its better understood bladder counterpart. The principal offender is exposure to renally excreted carcinogenic polycyclic aromatic hydrocarbons. Smoking and occupational exposure through the paint, dye and rubber industry are estimated to account for 50% and 10% of tumours, respectively. Obesity, a common risk factor across many malignancies, has also been shown to be associated with TCC and related to 20% of new diagnoses in the UK. Importantly, those with a known UTUC are found to have a contralateral malignancy in 1-6% of cases [1,2].
Aside from environmental factors, genetic susceptibility plays its part in TCC formation, with the risk being twice as high in those who have an affected first-degree relative [4]. Lynch syndrome can be found in up to 9% of patients diagnosed with UTUC and Amsterdam criteria is used to screen patients for Lynch syndrome after a diagnosis of UTUC [2].
Multifocal and ureteral tumours were found to be independent predictors of disease progression and cancer specific mortality in multivariable analyses [5].
Investigations of UTUC
The workup for urothelial malignancy requires detailed and thorough investigation in order to facilitate both diagnosis and risk stratification.
Cystoscopy
Cystoscopy is the gold standard test for urothelial carcinoma of the bladder. It is an integral part of haematuria screening to rule out bladder malignancy through direct visualisation. It makes for a good screening modality as it is a quick and efficient procedure which can be done under local anaesthesia, having the additional advantages of having high levels of sensitivity and specificity. In contrast, the main challenge with regards to investigating UTUC is that there is no such simple, applicable for all outpatient procedure to make an accurate diagnosis in the same manner. Cystoscopy as a diagnostic tool for UTUC is limited to picking up concomitant bladder TCC and to guide further cross-sectional imaging in those patients with visible haematuria where a bladder cause has not been identified [2].
Urine cytology
The main methodology for cytology sample collection is through collecting voided urine which encompasses the entirety of the urinary tract, from renal pelvis to urethra. The key issue, when cytology is positive, is that this does not aid tumour localisation. To overcome this obstacle, selective washouts from the ureter may be used during ureteroscopy. Upper urinary tract sampling has shown to be more sensitive than voided urine and allows for targeted management of the urinary tract [6]. This technique also overcomes the practical challenges of trying to obtain tissue biopsies from the upper urinary tract during ureteroscopy.
Urine cytology for both UTUC and LTUC, is highly specific but the sensitivity is correlated with the grade of the tumour, with a significantly higher sensitivity for high-grade TCC tumours than low-grade. Positive urinary cytology is usually associated with the presence of advanced disease. In a multicentre study, it was demonstrated that preoperative positive cytology for UTUC was found in 87% of non-organ confined disease and 83% of high-grade disease [7]. It has been shown that in low-grade lesions there is a sensitivity of less than 10%. For this reason, urinary cytology has limited utility in the diagnosis and follow-up of low- grade TCCs [8].
Urine cytology interpretation is variable and difficult to reproduce due to mimics and interpretation differences. Cytology has a place in the screening of high-grade TCCs as well as an adjunct in diagnosis, especially when cystoscopy proves negative. However, it is currently not sufficiently sensitive to be substituted for imaging and invasive testing in the diagnostic build-up.
Imaging for UTUC
CT urography remains the gold standard imaging for diagnosing UTUC. Through creation of a 3D construction of the urinary system any abnormalities of the urothelium caused by UTUC, shown by filling defects, can be assessed [8].
The European Association of Urology (EAU) recommend CT imaging during the urographic phase as it has been shown to be the most efficacious modality for the diagnosis of UTUC, with meta-analyses assessing the sensitivity and specificity demonstrating 92% and 95%, respectively [8]. CT urography is also necessary to enable staging of extramural disease progression to best guide overall management of the patient.
Use of contrast CT may be limited in individuals with impaired renal function or allergies to the iodinated contrast and, in such cases, MR urogram is indicated [2]. Imaging may not be able to distinguish between UTUCs and radiolucent stones, blood clots and extrinsic vascular compression which may have similar urographic appearances and require ureteroscopy for differentiation.
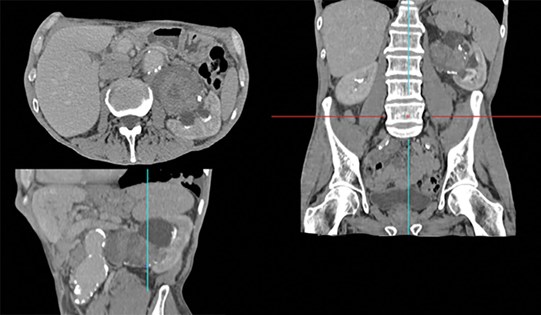
Figure 1: Reconstructed CT scan in urographic phase showing a large UTUC in the renal pelvis. This image also highlights the surgical challenge that can be present in such cases like the associated aortic aneurysm anterior to the UTUC. A reconstructed image is important in planning the treatment.
Diagnostic ureteroscopy and biopsy
Retrograde studies and ureterorenoscopic (URS) evaluations enable a full assessment of tumour morphology, appearance and focality. In addition, medical imaging and biopsies can be taken of any abnormal urothelium. Both rigid and flexible ureteroscopes can be used depending on the lesion location.
A biopsy is needed to grade and stage UTUC to enable risk stratification of low- and high-grade disease. The main difficulty of URS arises from the anatomy of the ureter complicating retrograde access and thus making sampling technically difficult. Grade of the tumour in these biopsies is a helpful surrogate for predicting invasive nature of the disease where G1 is non-invasive in close to 100% while G3 is likely to predict invasive disease. G2 is a mixed bag and needs to be used in conjunction with urine cytology and imaging characteristics to predict the invasive nature. pTa in these biopsies have been upstaged in up to 45% in final histology in RNU specimens while a pT1 is a more accurate prediction which is hardly upstaged.
Sampling issues lead to poor accuracy in risk stratifying UTUC patients and hence minimally invasive procedures being performed on those incorrectly labelled low-risk disease. It is crucial to incorporate all the diagnostic information to arrive at the risk stratification as tumour grade and stage correlates to disease-free survival, tumour progression and overall survival [6,9].
Management
RNU has been the gold standard in management of UTUC. However, this is a complex procedure with both short- and long-term morbidity. The association of loss of nephron units that leads to chronic kidney disease (CKD) and associated long-term morbidity has led to the quest of alternative renal sparing management. Just as in renal cell carcinoma (RCC) where a renal sparing approach has been shown to reduce morbidity from CKD, the search for alternatives to RNU has led to development of renal sparing treatments for UTUC. Accurate evaluation of stage and risk is important in determining the feasibility of minimally invasive techniques to maintain kidney function whilst maintaining good oncological outcomes [2,9].
Risk stratification [2]
Low risk: Unifocal, <2cm, low grade on URS biopsy and no invasive features on CT urography.
High risk: Multifocal disease, tumour size >2 cm, high-grade cytology, high-grade URS biopsy, local invasion on CT, hydronephrosis, previous radical cystectomy for high-grade bladder cancer, variant histology.
Low-risk UTUC
For low-risk UTUC, fresh emphasis has been placed on kidney sparing surgery due to endoscopic approaches having a safer morbidity profile than radical surgery and offering equivalent oncological outcomes [2]. The techniques utilised mainly include endoscopic ablation, segmental ureteric resection, and upper urinary tract instillation of topical agents. This can also be considered in cases where kidney function must be preserved due to poor pre-existing renal function or a solitary functional kidney [6,9]. The principal procedural complications of ureteroscopic management include perforation and ureteric stricture formation which would require stenting or nephrostomy insertion. Another important complication is intravesical recurrence of disease due to local seeding of disease. Recent evidence has shown the importance of a ureteral access sheath to mitigate this risk [10]. The main challenge with the endoscopic ablation approach such as with laser is recurrence and incomplete ablation due to issues with access and visualisation [9]. This, combined with the aforementioned issue of suboptimal risk categorisation and seeding, leads to minimally invasive procedures being performed with the knowledge that the patient is balancing preservation of renal function against higher rates of recurrence. A high degree of compliance is needed when such an approach is undertaken.
A 20-year, single-centre study of endoscopic management of UTUC from Edinburgh, demonstrated recurrence of disease in 68% of cases, 19% of which eventually required radical nephroureterectomy. This was in patients who had an intense follow-up regime with a median length of 54 months (range 1-223) [11].
This highlights the need for a patient having kidney-sparing procedures to agree to a rigorous follow-up regime involving frequent and continuous cytology, CT imaging and ureteroscopies and the understanding that, despite this, they may ultimately require an RNU [6,9].
Recently the US Food & Drug Administration (FDA) approved the use of JELMYTO®, a topical chemoablation using Mitomycin-containing thermal gel. This was following a phase three, single-arm trial using 24 sites across the USA and Israel. The dose of JELMYTO instilled is 4mg per ml via ureteral catheter or a nephrostomy tube, the total instillation volume being based on volumetric measurements using pyelography, not exceeding 15ml (60mg of mitomycin), once a week for six weeks. For patients with a complete response three months after JELMYTO initiation, instillations may be administered once a month for a maximum of 11 additional instillations. This showed promising results with 59% of the patients who received at least one instillation having a complete response. However, the side-effect profile should be borne in mind when undertaking this option. In the OLYMPUS trial, ureteric obstruction and stenosis occurred in 41% of the cases, urinary tract infection 32%, haematuria 31% and renal function impairment in 20% of the study group [12]. Due to UTUC usually presenting in the older age group, JELMYTO could prove a suitable therapeutic option rather than RNU. Other aqueous agents (Gemcitabine and Bacillus Calmette-Guerin (BCG)) are currently being explored in animal models [13].
High-risk UTUC
The gold-standard management of high-risk UTUC is RNU with bladder cuff excision, lymph node dissection and single dose intravesical chemotherapy. Unlike low- grade disease, adjuvant platinum-based chemotherapy has a proven efficacy for disease-free survival in high-grade UTUC and should be used in lymph node positive and muscle invasive disease, however this is limited by inability to deliver full-dose regimens post RNU [14]. There is also strong evidence that this postoperative chemotherapy should be extended for bladder instillation, using mitomycin C, to reduce intravesical disease [15]. In select patients with high-risk disease in the distal ureter, such as solitary kidney or with compromised renal function, segmental resection and reimplantation may be undertaken. However, it has to be done on an individual basis and not compromise oncological outcomes. A similar approach of BCG instillation for CIS in upper tract is still in its infancy.
Distal ureter management in nephroureterectomy
Various approaches to lower ureter management during nephroureterectomy have been undertaken. An endoscopic detachment of the lower ureter for ‘rip and pluck’, endoscopic resection of intramural ureter, open or robotic excision of the cuff of bladder by either transvesical or complete retroperitoneal stripping approach have all been undertaken. Though the gold standard is removal of a bladder cuff, there is no randomised study to compare the various techniques to manage the distal ureter.
References
1. Saginala K, Barsouk A, Aluru J, et al. Bladder cancer: epidemiology, risk factors, diagnosis, treatment. Medical Sciences 2020;8(1):1-17.
2. Rouprêt M, Babjuk, Burger M, et al. EAU Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma 2022
https://uroweb.org/guidelines/
upper-urinary-tract-urothelial-cell-carcinoma
[accessed 12 May 2022].
3. van Doeveren T, van de Werken HJG, van Riet J, et al. Synchronous and metachronous urothelial carcinoma of the upper urinary tract and the bladder: Are they clonally related? A systematic review. Urol Oncol 2020;38(6):590‑8.
4. Burger M, Catto JWF, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013;63(2):234-41.
5. Wu Y, Dong Q, Liu L, et al. The impact of tumour location and multifocality on prognosis for patients with upper tract urothelial carcinoma: a meta-analysis. Sci Rep 2014;4:6361.
6. Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 2012;110(5):614-28.
7. Brien JC, Shariat SF, Herman MP, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 2010;184(1):69-73.
8. Zhu C-Z, Ting H-N, Ng K-H, Ong T-A. A review on the accuracy of bladder cancer detection methods. J Cancer 2019;10(17):4038-44.
9. Raman J, Shore ND. Management of low-grade upper tract urothelial carcinoma: an unmet need. Rev Urol 2020;22(1):1-8.
10. Douglawi A, Ghoreifi A, Lee R, et al. Bladder recurrence following diagnostic ureteroscopy in patients undergoing nephroureterectomy for upper tract urothelial cancer: is ureteral access sheath protective? Urology 2022;160:142-6.
11. Cutress ML, Stewart GD, Wells-Cole S, Phipps S, Thomas BG, Tolley DA. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int 2012;110(11):1608-17.
12. Kleinmann N, Matin SF, Pierorazioet PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol 2020;21(6):776-85.
13. Patel SR, Lerner SP. Novel thermo-sensitive hydrogel therapy for low-grade upper tract urothelial carcinoma. J Urol 2021;206(2):191-3.
14. Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020;395(10232):1268-77.
15. O’Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol 2011;60(4):703-10.
TAKE HOME MESSAGE
-
Diagnosis and risk-stratification of UTUC require CT imaging, endoscopic evaluation, and cytology or histopathology.
-
Multifocality, tumour grade and stage at diagnosis are the key factors that determine the outcome in this disease.
-
Multifocality and ureteral tumours are independent predictors of disease progression and cancer specific mortality. They are associated with poor outcomes.
-
Accurate evaluation of grade and stage is important in determining the feasibility of endourological techniques.
-
Endourological techniques offer a renal sparing novel approach to low-grade, low-risk disease whilst maintaining good oncological outcomes.
-
Radical nephroureterectomy with bladder cuff excision remains the gold standard management of high-risk disease.