Prostate cancer is common with over 52,300 new cases diagnosed annually in the UK; this is expected to rise by 15% between 2023-2025 and 2038-2040 [1].
Radical prostatectomy continues to be the most common form of radical treatment for men with localised prostate cancer and a life expectancy greater than 10 years. The 10-year prostate cancer specific survival rates following radical prostatectomy approach 99% [2]. As survival from prostate cancer rises, it’s becoming increasingly important to provide management strategies for the sequelae of prostate cancer treatment. The side effects following cancer treatments may be associated with a decline in a patient’s quality of life.
The most reported side effects following prostate cancer treatment include erectile dysfunction and urinary incontinence. However, there are several other sexual problems often referred to as ‘neglected sexual side effects’ (NSSE). These include orgasmic dysfunction, ejaculatory dysfunction, libido loss and penile deformity and shortening. The discussion of sexual dysfunction is often difficult for patients to initiate with their clinician or with their partner(s). This may lead to feelings of isolation, depression, relationship dysfunction and psychological distress. In addition, the focus of discussions on sexual dysfunction is often based on heterosexual vaginal sexual intercourse. However, it is estimated that one in eight men who engage in anal sexual intercourse will be diagnosed with prostate cancer during their lifetime [3]. This patient group may experience different sexual problems which may require different solutions.
In this review, we will discuss the types of sexual dysfunctions men may experience and some strategies for their management.
Patient experiences of support for sexual dysfunction
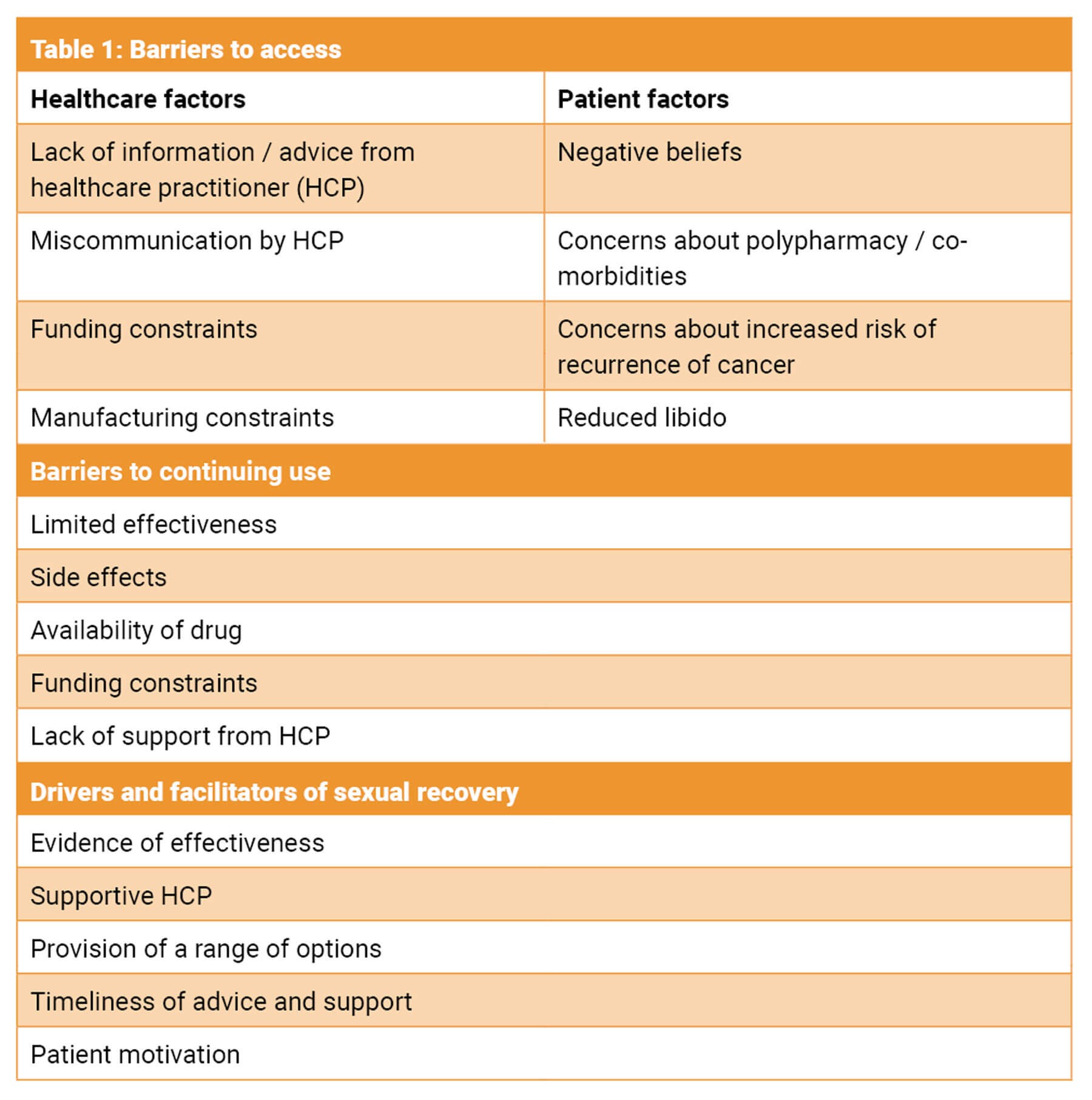
Prostate cancer survivors often report a reduction in sexual intimacy and impaired sense of masculinity after treatment; this may also be experienced by their partners [4]. The life after prostate cancer diagnosis (LAPCD) study was a UK study which recruited over 35,000 men [5]. This study found that 41.4% were offered medications / aids to assist with erectile dysfunction, 22.6% were offered devices and only 14.8% were referred for specialist services. This was duplicated in a more recent survey by Watson et al. where only 48.3% were offered any intervention to support sexual wellbeing [6]. The survey found that 39% reported poor or very poor sexual function which they considered a moderate or large problem [6]. The proportion offered any intervention varied with the type of prostate cancer treatment and was inversely proportional to rising age and the number of patient co-morbidities. Men who had been treated surgically were the most likely to be offered intervention. The intervention men found most helpful was referral to specialist services, but they were most likely to try medications [6]. However, referral to specialist services was seldom offered. Table 1 reveals some common themes from patients’ free text [6].
Couples’ therapy and lifestyle management
Men should be appropriately counselled prior to prostate cancer treatment about possible side effects; this should ideally be with their partner. There is good evidence that erectile function may be improved with lifestyle changes, and this may help to optimise sexual function prior to prostate cancer treatment [7].
Sex involves more than one person and therefore both the patient and his partner(s) may be affected adversely by his sexual dysfunction. A recent study revealed that 64% of partners reported sexual problems and difficulty adjusting to sexual side effects [8]. It is therefore important that the partner is included in all sexual dysfunction consultations. Common negative emotions include frustration, loss of familiar sexual interaction, fear, and anger [9]. Sexual dysfunction may be a significant relationship stressor with the patient feeling isolated and embarrassed and the partner feeling unattractive or invisible. This may result in poor communication between the couple and relationship conflict. We would recommend the following strategies based on the study by Li et al. [9]:
- Emphasis on couple communication to preserve and expand sex life and discuss sexual dysfunction.
- Redefining physical intimacy: this may involve non-intercourse driven intimate touching.
- Incorporating sexual aids (see section later).
- Sexual exploration.
Couples’ counselling initiated early (<3 months) after surgery, may improve sex life, and nurse-delivered telephone counselling, peer support and traditional psychosexual therapy may all be beneficial [10]. An important caveat to this is some men may not have a regular sexual partner at the time of prostate cancer treatment. The side effects may discourage them from seeking out both support and treatment as well as seeking out future sexual partner(s). However, they should be offered the same degree of therapy / lifestyle management.
Sexual aids including vacuum erection device
Sexual aids are any objects / devices which may be used to facilitate or enhance human sexual pleasure or function [11]. There are a wide range of these devices now available for patients to purchase on the internet or in specialist shops. In addition, some are now being used medically for sexual dysfunction i.e. vacuum erection device (VED). It’s important to broach this topic with sensitivity as some patients may find it unacceptable due to religious, cultural or relationship beliefs. Table 2 illustrates the main types of devices, their indications, and possible complications [11].
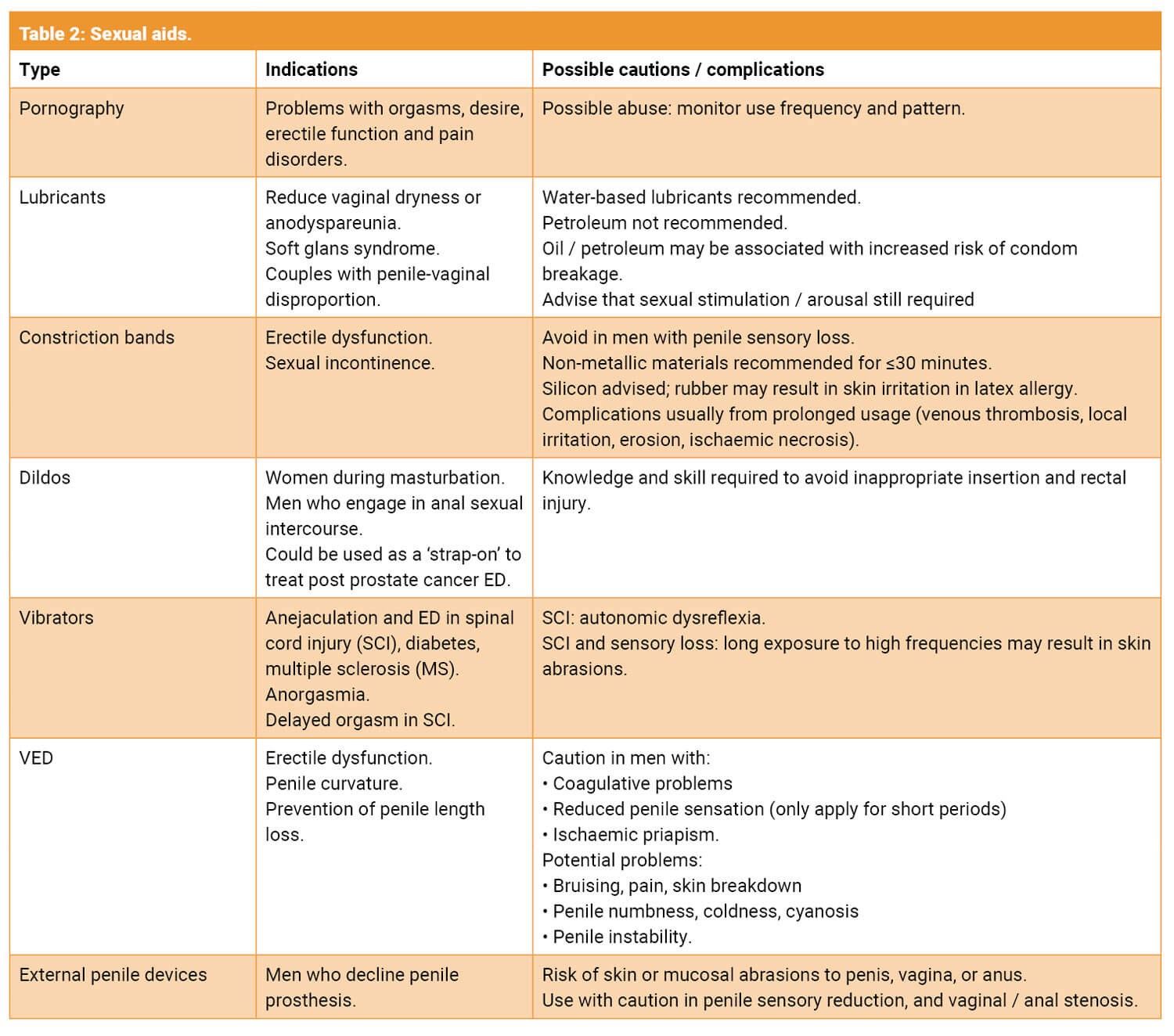
VEDs are probably the most used device in andrological practice. These devices increase the amount of blood in the penis by creating a negative pressure with a band then trapping the blood in the penis to create an erection sufficient for sexual intercourse. The erection is not dependant on functional nerves or a fully intact vascular blood supply [12]. This makes the device ideal for men following prostate cancer treatment, especially those who decline or are not suitable for other modes of treatment. A systematic review in 2018 revealed that there was a significant improvement in erectile function and successful intercourse when VED was implemented early [12]. In addition, early use could improve penile length. The optimal time to initiate use was one to four months after surgery [12]. Side effects were minor and infrequent.
Erectile dysfunction
Erectile dysfunction (ED) is one of the most common complications following prostate cancer treatment and has a negative impact on quality of life. This may be caused by injury to the cavernous nerves by either incomplete nerve-sparing surgery, or neuropaxia due to stretching, thermal injury and / or direct trauma to the nerve [13]. Arterial or venous insufficiency post prostatectomy may induce a hypoxic environment leading to corporal fibrosis, through collagen deposition and cavernous smooth muscle cell apoptosis.
It is important to note that ED is common following other treatment modalities for prostate cancer. The presentation is often more delayed with radiotherapy and may be associated with low libido when it is combined with androgen deprivation therapy (ADT).
There is a consensus that ED treatment should start early, to prevent corporal fibrosis and increase reoxygenation to the penile tissues, as the incidence of veno-occlusive dysfunction increases in a time-dependent fashion post radical prostatectomy [14].
Across studies, improvement in postoperative potency rates favours bilateral nerve-sparing technique, over unilateral, over non-nerve-sparing surgery and robotic assisted over open retropubic radical prostatectomy [13].
Assessment of ED
Management of a prostatectomy patient’s functional outcomes begins with preoperative counselling. It is important to try and involve partners in this process if possible. Erectile dysfunction may be permanent or temporary, and while most patients have a gradual improvement in erectile function usually with treatment, few return to their preoperative baseline function.
For baseline measurements, tools such as the International Index of Erectile Function (IIEF) or Sexual Health Inventory for Men (SHIM) can be used both pre- and postoperatively. This enables patient expectations to be assessed, and for the patient and clinician to discuss realistic expectations from treatment. Timescale is also important, as in most cases, if functional recovery is achieved, this will be between 12 and 24 months postoperatively [15]. During preoperative counselling additional side effects such as ejaculatory and orgasmic changes, reduction in penile length and changes in libido should also be discussed.
As with any patient presenting with ED, a thorough medical history should be taken, body mass index (BMI) calculated, and other risk factors assessed.
ED management
Management of ED following prostate cancer treatment should include phosphodiesterase-5 inhibitors (PDE5i), VED, intracavernosal or intraurethral prostaglandins, or combination therapy. Surgical treatment with insertion of penile prosthesis is reserved for patients who have failed, are unsuitable for medical therapy or wish for definitive treatment.
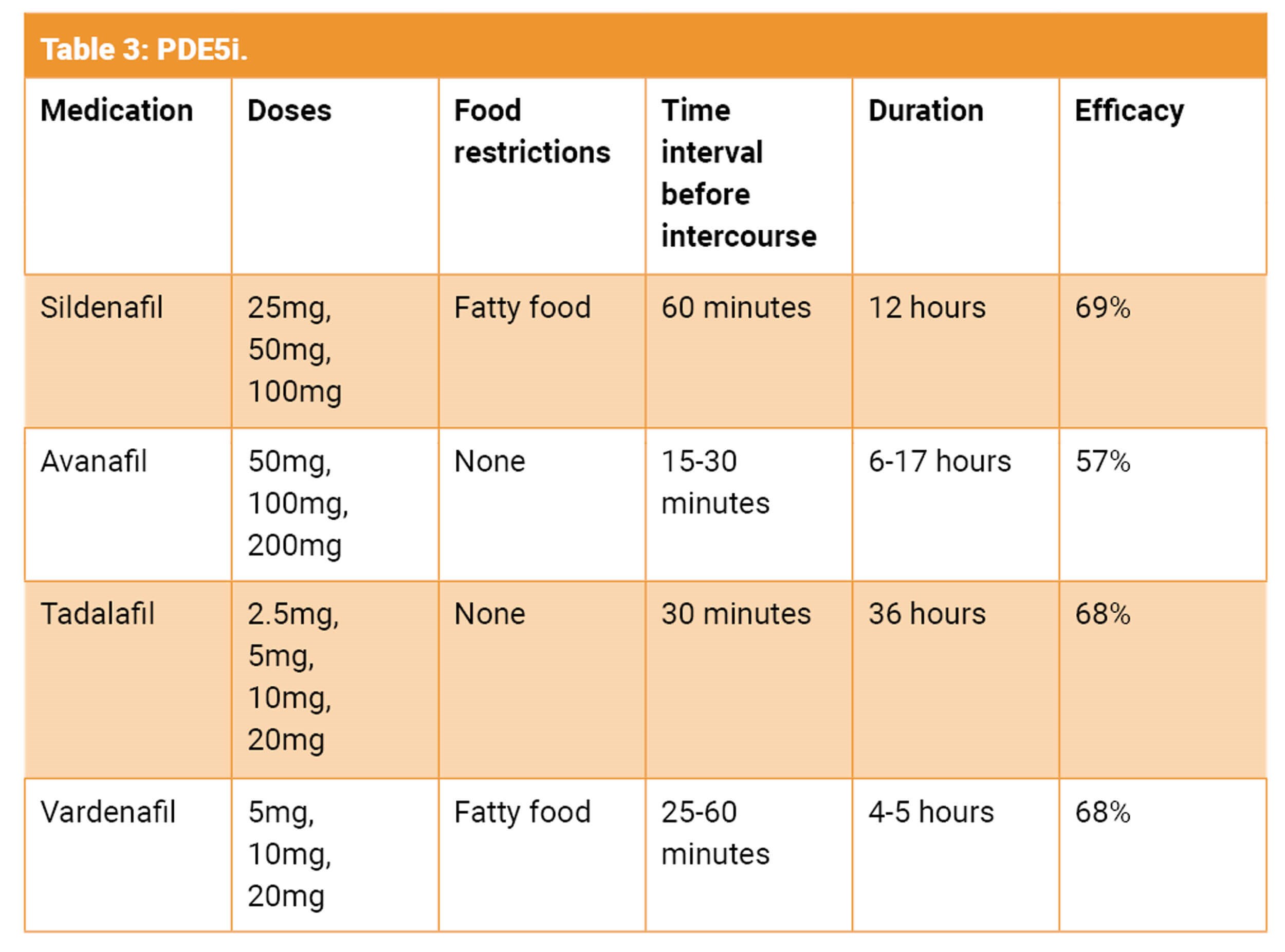
Although there is clear evidence that the use of early PDE5i improves erectile function following prostate cancer treatment, the data is very conflicting and unclear with regards to the optimal regime [14,16]. This is probably due to differences in ED definition, patient characteristics, follow-up time and the type of regime utilised. The factors associated with good PDE5i response include age, baseline erectile function, quality of nerve-sparing technique and surgeon experience [17]. There is currently no consensus about the use of daily versus on-demand PDE5i or any specific penile rehabilitation protocol.
Safety concerns for PDE5i [16]
- Safe in men with ischaemic heart disease. However, assess cardiac fitness for sexual intercourse.
- Absolute contraindication with nitrates. Patient must avoid nitrate for 24-48 hours if chest pain occurs after PDE5i use.
- Absolute contraindication with nicorandil.
- Use with caution with antihypertensives and α-blockers.
How to manage PDE5i non-responders [16]
It is estimated that approximately a third of men will fail to respond to PDE5i. However, before labelling them treatment failures, it is important to ensure the following:
- Sufficient trial with licensed medication. A trial of six attempts with a drug is adequate.
- Sufficient time interval has been allowed before attempt at sexual activity.
- Sufficient drug dose utilised.
- Use of sexual stimulation.
- Avoidance of use with fatty food with sildenafil and vardenafil.
In patients who are true treatment failures, second-line treatment options should be discussed with the patient. This should include advantages and disadvantages of each treatment and referral for specialist services with an andrologist. The European Association of Urology guidelines suggest that part of the first-line approach for treatment could involve either switching the type of PDE5i, combining a short-acting PDE5i (i.e. sildenafil) to daily tadalafil or combining a PDE5i with a VED [16]. In addition, hypogonadal men may have better response if treated with a PDE5i and testosterone replacement therapy (TRT). Although there is no evidence that TRT increases the risk of prostate cancer or its relapse, its use should be implemented with a specialist [16]. The current guidelines recommend caution in prostate cancer survivors, avoidance in advanced cancer and treatment initiation at least one year after follow-up and with a prostate specific antigen (PSA) <0.001ng/mL [16].
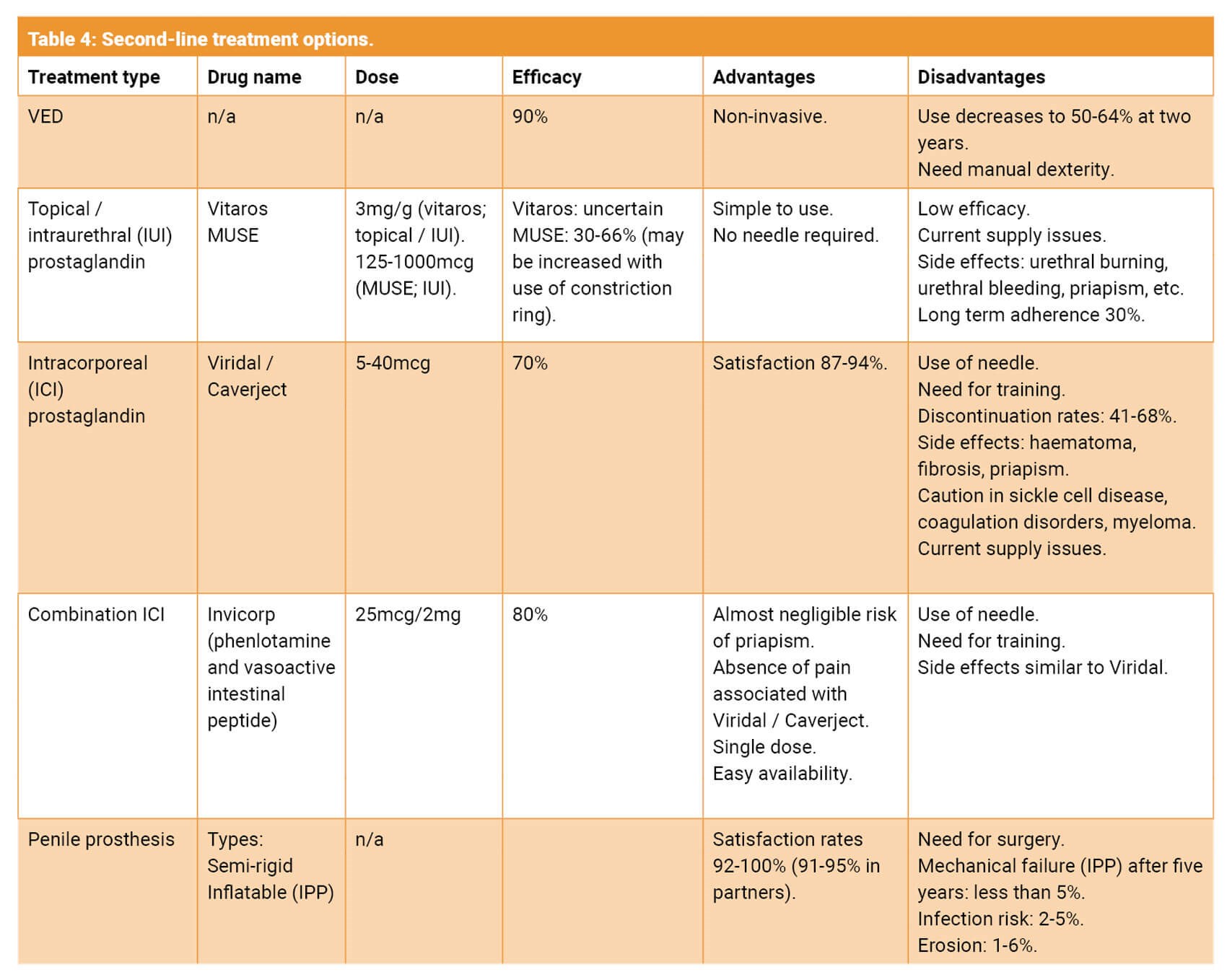
Table 4 illustrates the second-line treatment options. It is important to mention on-going manufacturing issues with many of the second-line treatments which has affected patients’ ability to obtain these medications.
Sexual incontinence
Sexual incontinence consists of arousal incontinence (AI) and climacturia [18]. AI is defined as involuntary urinary incontinence during arousal or sexual intercourse prior to orgasm. However, climacturia occurs in association with orgasm. The exact aetiology is poorly understood but AI may be due to deficiency of the external sphincter and pelvic floor relaxation occurring after radical prostatectomy [18,19]. Climacturia which has been reported after both radical prostatectomy and radiotherapy may be associated with a reduction in the functional urethral length and bladder neck incompetence [19].
There is reported prevalence ranging from 15.7–93% for climacturia and 29–82% for AI [3,19-22]. The rates reported after radiotherapy are significantly lower [23]. Both climacturia and AI are associated with moderate to significant bother with resultant avoidance of sex, reduction in erection confidence and quality of life [18,22,23]. Most men experienced small volume leakages infrequently [22,23]. Although symptoms have been reported to present in most men as early as three months following surgery, there was a trend towards improvement over time [14,24,25].
The proposed risk factors for sexual incontinence are prior radiotherapy or prostatectomy, younger age, erectile dysfunction, and worsening stress urinary incontinence [22,23]. Bothersome symptoms were associated with the frequency and quantity of leakage, partner bother, stress urinary incontinence and a shorter patient relationship [22,23]. Robotic assisted radical prostatectomy may be an independent predictor of faster resolution of symptoms of climacturia with a nerve-sparing approach being associated with a reduction in the risk of bother [23,26].
There are no definitive treatment options, however suggested lifestyle modifications to reduce symptoms include voiding prior to intercourse or to use condoms. Pelvic floor muscle exercises (PFME) and constriction device at the penile base have shown some encouraging improvements in small studies [27].
Surgical options for patients with daytime urinary incontinence include a urethral sling or artificial urinary sphincter, dependent on severity and patient preference, and these have been shown to improve symptoms in patients with concomitant climacturia [28].
A promising surgical option for those suffering from erectile dysfunction, mild urinary incontinence and climacturia, is the Mini-Jupette graft at the time of insertion of an inflatable penile prosthesis. This involves laying the graft over the proximal urethra and suturing it to the corporal cavernosum bilaterally. This provides gentle urethral compression when the prosthesis is inflated thereby preventing urinary leakage. A multi-centre cohort study demonstrated subjective improvement in symptoms of climacturia in 92.8% of patients at a mean follow up of five months [29].
Ejaculatory dysfunction: anejaculation
Anejaculation is an inevitable consequence after radical prostatectomy, during which the prostate and seminal vesicles are removed and the vas deferens are disconnected. A study by Deveci et al., which surveyed men postoperatively about their expectations for recovery, reported that nearly half of patients were unaware they would develop anejaculation following surgery [30].
Orgasmic disturbances: altered orgasmic sensation, anorgasmia, dysorgasmia
Radical prostatectomy may result in the following effects on orgasm:
- Absence (anorgasmia)
- Reduce or altered intensity
- Painful orgasm (dysorgasmia) [3,10].
Orgasmic dysfunction, especially anorgasmia, may be associated with a significant reduction in psychosexual wellbeing and relationship health [10]. The aetiology of these changes is not well understood but a possible theory is that neuropraxia may lead to anorgasmia or dysorgasmia. Another possible explanation for dysorgasmia is the bladder neck contraction occurring during orgasm leading to spasm at the vesicourethral anastomosis or pelvic floor dystonia [10,24]. The latter theory is supported by the improvement of dysorgasmia following tamsulosin [10]. Carbergoline (dopamine agonist) has been used off-licence in the treatment of anorgasmia, though in the study suggesting its efficacy it was a mixed cohort of patients where less than 20% had undergone radical prostatectomy. Vardenafil has also been used to treat orgasmic function following radical prostatectomy [10].
The prevalence of orgasmic dysfunction has been reported as 5–78% for anorgasmia, 41–60% for altered orgasmic intensity and 10–14% for dysorgasmia [3,10]. Orgasmic dysfunction is associated with older patient age, non-nerve-sparing status, and severe urinary incontinence [10,31].
Penile anatomical changes
Penile shortening and deformity have been documented following both open and robotic-assisted radical prostatectomy. Most studies suggest a loss of penile length from open surgery of up to 2cm at 12 months, with men undergoing the robotic approach returning to baseline at 9–12 months [31]. The estimated incidence of deformity / curvature is 3.2–16% with an average curvature of 31 degrees at 13.9 months following surgery [32].
The suggested pathophysiology is that hypoxia to the corporal tissue due to arterial damage or neuropraxia leads to smooth muscle loss and penile shortening. This is one rationale for starting early penile rehabilitation to minimise this fibrotic process.
Nerve-sparing surgery, recovery of erectile function and younger age were predictors of retaining length [24]. Younger, Caucasian men were also more likely to develop penile curvature with a nerve-sparing approach being marginally protective [32].
Medical management options used include tadalafil and irbesartan, to limit the reduction in penile length and circumference.
Management should follow the same pathways as penile curvature unrelated to prostate cancer treatment i.e., PDE5Is to manage concomitant erectile dysfunction, non-steroidal anti-inflammatory drugs (NSAID), VEDs and referral to specialist services for consideration of penile prosthesis +/- straightening procedures. The vacuum erection device has been used in several small studies, with a tendency towards this showing less reduction in penile length when compared to no treatment [33]. However, these outcomes were self-assessed retrospectively, and it is unclear if results were maintained long-term. In addition, the use of VED prior to penile prosthesis may increase penile length and girth thereby allowing for the implantation of a larger prosthesis and improved patient satisfaction [34].
References
1. What is prostate cancer? Cancer Researc UK
https://www.cancerresearchuk.org/
about-cancer/prostate-cancer/about
[last accessed 21 November 2023].
2. Hamdy FC, Donovan J, Lane A, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375(15):1415–24.
3. Röscher P, Sathiram R, Milios JE, van Wyk JM. Mapping the prevalence and use of questionnaires to detect the neglected sexual side effects after prostate cancer treatment: a scoping review. BMC Systematic Review 2022;11:2–12.
4. Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 2003;21:3777–84.
5. Downing A, Wright P, Hounsome L, et al. Quality of life in men living with advanced and localised prostate cancer in the UK: a population-based study. Lancet Oncol 2019;20:436–47.
6. Watson E, Wilding S, Matheson L, et al. Experiences of support for sexual dysfunction in men with prostate cancer: findings from a UK-wide mixed methods study. J Sex Med 2021;18:515–25.
7. Moyad MA, Barada JH, Lue TF, et al.; Sexual Medicine Society Nutraceutical Committee. Prevention and treatment of erectile dysfunction using lifestyle changes and dietary supplements: what works and what is worthless, part II. Urol Clin North Am 2004;31:259–73.
8. Grondhuis Palacios LA, den Ouden MEM, den Oudsten BL, et al. Treatment-related sexual side effects from the perspective of partners of men with prostate cancer. J Sex Marital Ther 2019;45:440–51.
9. Li R, Wittmann D, Nelson CJ, et al. Unmet sexual health needs of patients and female partners following diagnosis and treatment for prostate cancer. J Sex Med 2022;19:1797–803.
10. Dubbelman Y, Wildhagen M, Schröder F, et al. Orgasmic dysfunction after open radical prostatectomy: clinical correlates and prognostic factors. J Sex Med 2010;7:1216–23.
11. Miranda EP, Taniguchi H, Cao DL, et al. Application of sex aids in men with sexual dysfunction: a review. J Sex Med 2019;16:767–80.
12. Qin F, Wang S, Li J, et al. The early use of vacuum therapy for penile rehabilitation after radical prostatectomy: systematic review and meta-analysis. Am J Mens Health 2018;12(6):2136–43.
13. Lima TFN, Bitran J, Frech FS, Ramasamy R. Prevalence of post-prostatectomy erectile dysfunction and a review of the recommended therapeutic modalities. Int J Impot Res 2021;33(4):401–9.
14. Saleh A, Abboudi H, Ghazal-Aswad M, et al. Management of erectile dysfunction post-radical prostatectomy. Res Rep Urol 2015;7:19–33.
15. Mulhall JP, Bella AJ, Briganti A, et al. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med 2010;7(4 Pt 2):1687–98.
16. European Association of Urology. Sexual and Reproductive Health Guidelines 2023.
https://uroweb.org/guidelines/
sexual-and-reproductive-health
[last accessed 21 November 2023].
17. Briganti A, Gallina A, Suardi N, et al. Predicting erectile function recovery after bilateral nerve sparing radical prostatectomy: a proposal of a novel preoperative risk stratification. J Sex Med 2010;7(7):2521–31.
18. Salter CA, Bach PV, Katz D, et al. The relationship and psychosocial impact of arousal incontinence after radical prostatectomy. J Sex Med 2020;17(1):94–8.
19. Bach PV, Salter CA, Katz D, et al. Arousal incontinence in men following radical prostatectomy: prevalence, impact and predictors. J Sex Med 2019;16(12):1947–52.
20. Mykoniatis I, van Renterghem K, Sokolakis I, et al. Climacturia: a comprehensive review assessing pathophysiology, prevalence, impact, and treatment options regarding the “leak of pleasure”. Int J Impot Res 2021;33(3):259–70.
21. Jain R, Mitchell S, Laze J, Lepor H. The effect of surgical intervention for stress urinary incontinence (UI) on post-prostatectomy UI during sexual activity. BJU Int 2012;109:1208–12.
22. Salter CA, Bach PV, Miranda E, et al. Bother associated with climacturia after radical prostatectomy: prevalence and predictors. J Sex Med 2020;17(4):731–36.
23. Jimbo M, Alom M, Pfeifer ZD, et al. Prevalence and Predictors of Climacturia and Associated Patient/Partner Bother in Patients With History of Definitive Therapy for Prostate Cancer. J Sex Med 2020;17(6):1126–32.
24. Nolsøe AB, Jensen CFS, Østergren PB, Fode M. Neglected side effects to curative prostate cancer treatments. Int J Impot Res 2021;33(4):428–38.
25. Mitchell SA, Jain RK, Laze J, Lepor H. Post-prostatectomy incontinence during sexual activity: a single center prevalence study. J Urol 2011;186(3):982–5.
26. Capogrosso P, Ventimiglia E, Cazzaniga W, et al. Orgasmic Dysfunction after Radical Prostatectomy. World J Mens Health 2017;35(1):1–13.
27. Mehta A, Deveci S, Mulhall JP. Efficacy of a penile variable tension loop for improving climacturia after radical prostatectomy. BJU Int 2013;111:500–4.
28. Jain R, Mitchell S, Laze J, Lepor H. The effect of surgical intervention for stress urinary incontinence (UI) on post-prostatectomy UI during sexual activity. BJU Int 2012;109(8):1208–12.
29. Yafi FA, Andrianne R, Alzweri L, et al. Andrianne Mini-Jupette graft at the time of inflatable penile prosthesis placement for the management of post-prostatectomy climacturia and minimal urinary incontinence. J Sex Med 2018;15(5):789–96.
30. Deveci S, Gotto GT, Alex B, et al. A survey of patient expectations regarding sexual function following radical prostatectomy. BJU Int 2016;118(4):641–5.
31. Vanderhaeghe D, Albersen M, Weyne E. Focusing on sexual rehabilitation besides penile rehabilitation following radical prostatectomy is important. Int J Impot Res 2021;33(4):448–56.
32. Tal R, Heck M, Teloken P, et al. Peyronie’s disease following radical prostatectomy: Incidence and predictors. J Sex Med 2010;7:1254–61.
33. Egydio PH, Kuehhas FE. The multiple-slit technique (MUST) for penile length and girth restoration. J Sex Med 2018;15(2):261–9.
34. Tsambarlis PN, Chaus F, Levine LA. Successful placement of penile prostheses in men with severe corporal fibrosis following vacuum therapy protocol. J Sex Med 2017;14:44–6.
Declaration of competing interests: None declared.








