Diffusion weighted imaging and contrast enhanced imaging
Introduction
Magnetic resonance imaging (MRI) is widely used in localisation, staging and post-treatment follow-up of prostate cancer. In the previous issue, we discussed the usefulness of MRI in depicting prostate anatomy and pairing conventional T1 and T2-weighted imaging (T2WI) to localise and stage prostate tumours. T1WI and T2WI alone have limited sensitivity and specificity, as there is considerable overlap in signal characteristics in benign conditions, such as prostatitis, benign prostatic hyperplasia (BPH), post-radiation fibrosis and changes related to hormone therapy [1].
More advanced MR techniques, such as diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI are used in conjunction with T2WI to image the prostate. These techniques allow discrimination of functional parameters in normal vs. tumoural tissues, thus improving diagnostic accuracy in prostate cancer detection [1].
Basics of diffusion-weighted imaging
Molecular diffusion is a natural consequence of Brownian motion, the innate random movement of molecules suspended in a fluid due to constant thermal agitation [2]. This was originally described by botanist Robert Brown in 1827 when he noticed that grains of pollen suspended in water were in constant, random motion [3]. Although the average displacement of the molecules over time is zero, there is a non-zero probability that the molecule will be displaced from its point of origin at any given point in time [2]. This concept is used to derive the diffusion coefficient of the fluid. For example, the diffusion coefficient for pure water is about 2.2 x 10-3 mm2/s [2]. Soft tissues will have a reduced diffusion coefficient due to increased density of molecules causing reduced movement of extracellular water molecules. Cell membrane permeability also affects the free diffusion of water, thus also affecting the diffusion coefficient. As such, when applying this model to living tissues, an ‘apparent diffusion coefficient’ or ADC is used rather than the diffusion model of pure water.
MR DWI exploits the signal loss associated with the random thermal motion of water molecules in living tissues in the presence of a magnetic field gradient [2]. Freely moving water molecules lose signal in an applied magnetic field whereas particles with restricted movement (i.e. extracellular water molecules in malignant tissues of high cellularity) are unaffected by the magnetic field and retain their signal [4]. Thus, malignant tissue will appear as high signal on the DWI sequence.
Post-processing of diffusion-weighted images allows generation of an ADC map from calculated apparent diffusion coefficients for the tissue being analysed. Highly cellular malignant tissues will demonstrate a reduction in ADC values, appearing as an area of decreased signal on the ADC map [5]. DWI has the advantage of rapid acquisition time, and is obtained without the use of intravenous contrast [5].
Use of DWI in prostate cancer
Peripheral zone tumours
On conventional T2WI, peripheral zone prostate carcinomas appear as a low signal area within the normally high signal peripheral zone. However, as described above, many noncancerous pathologies may also appear as low signal within the peripheral zone, lowering the specificity of T2WI alone in detecting prostate cancer. DWI can aid diagnosis, as prostate carcinoma demonstrates restricted diffusion due to the replacement of normal glandular architecture with tightly packed tumour cells and fibrotic stroma, thereby restricting the movement of water molecules [1].
Tumours appear as high signal areas on DWI and low signal areas on ADC map (Figure 1).
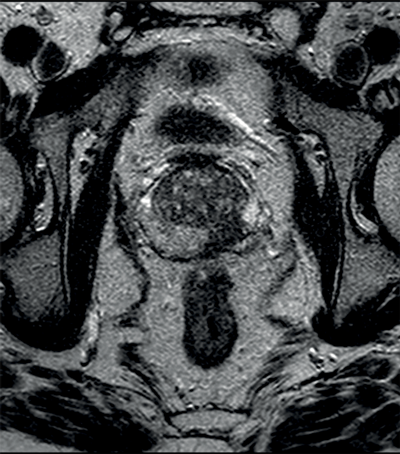
Figure 1a: Axial T2WI showing low signal left peripheral zone tumour.
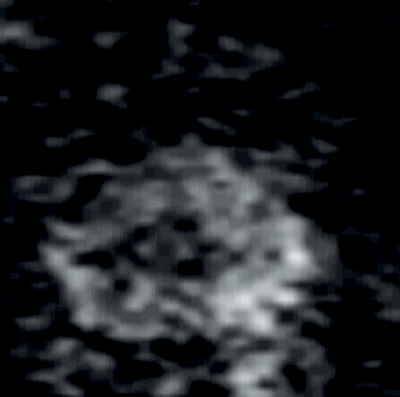
Figure 1b: DWI showing high signal at the site of tumour.
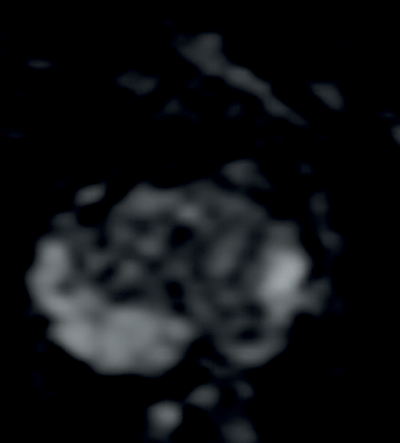
Figure 1c: ADC map with low signal seen confirming restricted diffusion.
Most studies evaluating DWI in prostate cancer have looked at tumours within the peripheral zone [5]. Combined T2WI and DWI have shown improved results in the detection of prostate cancer, yielding sensitivity and specificity rates of 45-89% and 61-97% respectively, compared with 74-85% and 57-95% respectively for DWI alone and 25-87% and 57-92% respectively for T2WI alone [5].
Transitional / central zone tumours
Transitional and central zone tumours are more difficult to evaluate using conventional T1 and T2WI as both normal and cancerous tissues in these areas demonstrate low signal intensity. Additionally, the normal central gland is commonly heterogeneous, making delineation of abnormal areas challenging. DWI may improve the detection and localisation of central / transitional zone tumours, as they also demonstrate restricted diffusion. Studies have shown that transitional zone tumours have lower ADC values than normal gland as well as BPH nodules [5,6] (Figure 2). The sensitivity for tumour detection in the transitional zone, however, still remains less than in the peripheral zone [5].
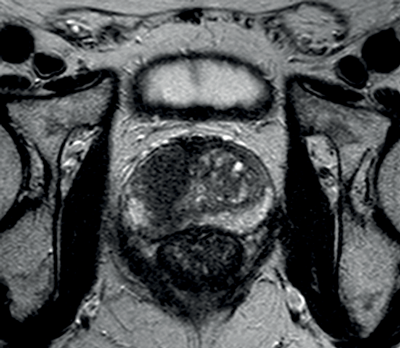
Figure 2a: Axial T2WI with low signal in the right central zone.
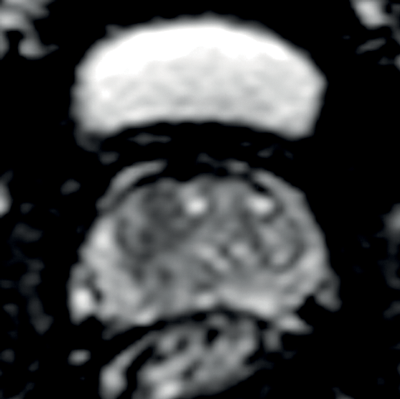
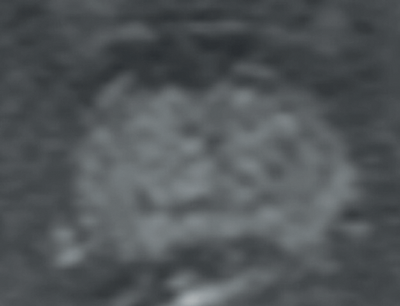
Figures 2b and 2c: DWI and ADC map confirm restricted diffusion at the site of tumour.
DWI and Gleason score
Several studies have shown a correlation between the pathological grade of prostate cancer – as described by the Gleason score – and the measured ADC values. Higher Gleason grading is associated with lower ADC values, most likely due to the lack of differentiation and higher stromal components as compared to the more organised architecture of low grade, well-differentiated tumours, which more closely resemble normal glandular tissue [7]. In a retrospective study of 57 patients with biopsy-proven prostate cancer, Woodfield et al. demonstrated that DWI may help to differentiate between low and intermediate-risk and low and high-risk prostate cancer, which may influence treatment options [8].
Dynamic contrast-enhanced imaging
Basis of dynamic contrast-enhanced MRI
Dynamic contrast-enhanced (DCE) MRI relies on tumour angiogenesis, or the propensity of malignant tissue to promote microvessel formation. This is attributed to both tissue hypoxia and tumoural expression of angiogenic factors, such as vascular endothelial growth factor and vascular permeability factor [7]. The result is budding and growth of a new network of blood vessels, which tend to be more permeable and disorganised than normal in vivo vessels [7]. There is increased interstitial space in cancerous tissue, which creates a greater differential concentration of contrast between plasma and the interstitium [1]. These factors contribute to characteristic enhancement patterns and pharmacokinetics of malignant tissue, which differ from that of normal tissue. Additionally, these attributes have been shown to be more exaggerated in prostate cancer than other benign hypervascular processes such as BPH [7].
Imaging technique
DCE MRI uses multiple, serial images which are obtained following the administration of contrast media, commonly a gadolinium-based compound. Contrast molecules pass from arteries to tumour microvessels and extravasate into the extravascular extracellular space within seconds. Gadolinium molecules in these areas shorten local tissue relaxation times, causing high signal on T1-weighted sequences. DCE sequences require fast bolus administration and rapid image acquisition techniques to ensure detection of enhancement [9]. Images are acquired before, during and after bolus injection.
Enhancement patterns of malignant tissue
DCE MRI uses a complex pharmacokinetic model to describe the accumulation of contrast molecules within the intravascular and extravascular spaces over time. From a qualitative perspective, we may understand that increased tumour vascularity and neovessel permeability leads to earlier, faster and more intense enhancement (‘wash-in’) of cancerous tissue when compared to the normal, or benign, prostatic tissue [7]. For similar reasons, there is more rapid ‘wash-out’ of contrast agent from malignant tissue, as compared to normal tissue, which continues to increase in signal during the first few minutes following contrast injection [9].
DCE MRI in prostate cancer
In prostate cancer, DCE MR follows the characteristic pattern of malignant enhancement with early, intense enhancement and rapid wash-out. It is most often correlated with T2-weighted and diffusion-weighted sequences for greater accuracy in detection (Figure 3).
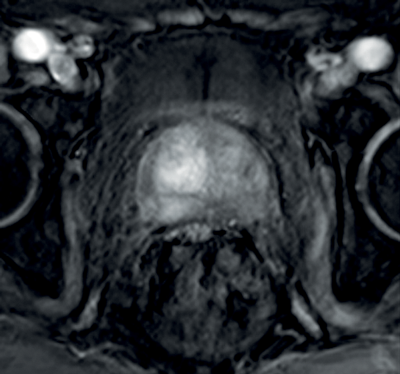
Figure 3a: DCE image of the same patient as Figure 2 showing
avid enhancement in the right central zone tumour.
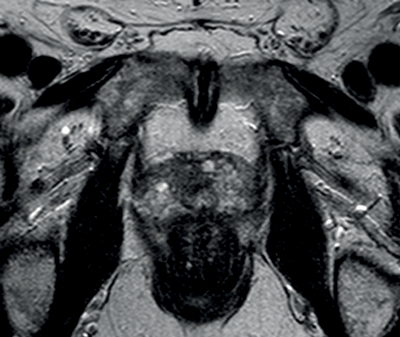
Figure 3b: T2WI showing tumour at the right apex.
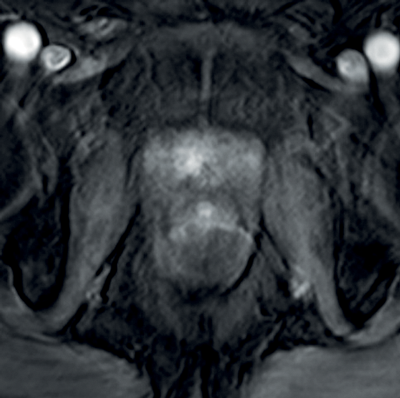
Figure 3c: Avid enhancement seen within the tumour.
One study demonstrated that correlating T2WI with DWI and DCE MR yielded a sensitivity of 83%, whereas the sensitivity of DCE MR alone was only 43% [9]. Furthermore, DCE MR has proven to be useful in the detection of tumour recurrence, where normal anatomical landmarks have been obliterated by treatment, making interpretation of T2WI alone difficult [9]. Particularly in cases of radical prostatectomy for prostate carcinoma, detection and localisation of recurrence is important as patients may benefit from further treatment (e.g. radiation therapy).
Conclusion
Multi-parametric MRI, including DWI and DCE MRI has gained increasing popularity in evaluating tumours of the prostate. Used in conjunction with conventional T1WI and T2WI, these techniques have demonstrated increased sensitivity and specificity in detection and localisation of primary prostatic carcinoma, as well as recurrence. Future advancements in functional MRI may further improve these statistics, thereby improving overall patient management and follow-up care.
References
1. Choi YJ, Kim JK, Kim N, et al. Functional MR Imaging of Prostate Cancer. RadioGraphics 2007;27(1):63-77.
2. Luypaert R, Boujraf S, Sourbron S, et al. Diffusion and perfusion MRI: basic physics. Eur J Radiol 2001;38:19-27.
3. Goncalves SI. Diffusion weighted MR Imaging. 2011; PowerPoint presentation from the Radiology Department of University Hospital Coimbra.
4. Gaillard F, Bashir U, et al. Diffusion weighted imaging.
[Accessed from www.radiopaedia.org]
5. Tan CH, Wang J, Kundra V. Diffusion weighted imaging in prostate cancer. Eur Radiol 2011;21(3):593-603.
6. Kayhan A, Fan X, Oommen J, et al. Multi-parametric MR imaging of transition zone prostate cancer: Imaging features, detection and staging. World J Radiol 2010;2(5):180-7.
7. Bonekamp D, Jacobs MA, El-Khouli R, et al. Advancements in MR Imaging of the Prostate: From Diagnosis to Interventions. RadioGraphics 2011;31(3):677-703.
8. Woodfield CA, Tung GA, Grand DJ, et al. Diffusion-Weighted MRI of Peripheral Zone Prostate Cancer: Comparison of Tumour Apparent Diffusion Coefficient with Gleason Score and Percentage of Tumour on Core Biopsy. Am J Roentgenol 2010;194:316-22.
9. Verma S, Turkbey B, Muradyan N, et al. Overview of Dynamic Contrast-Enhanced MRI in Prostate Cancer Diagnosis and Management. Am J Roentgenol 2012;198:1277-88.
Part 1 of this study can be found here






