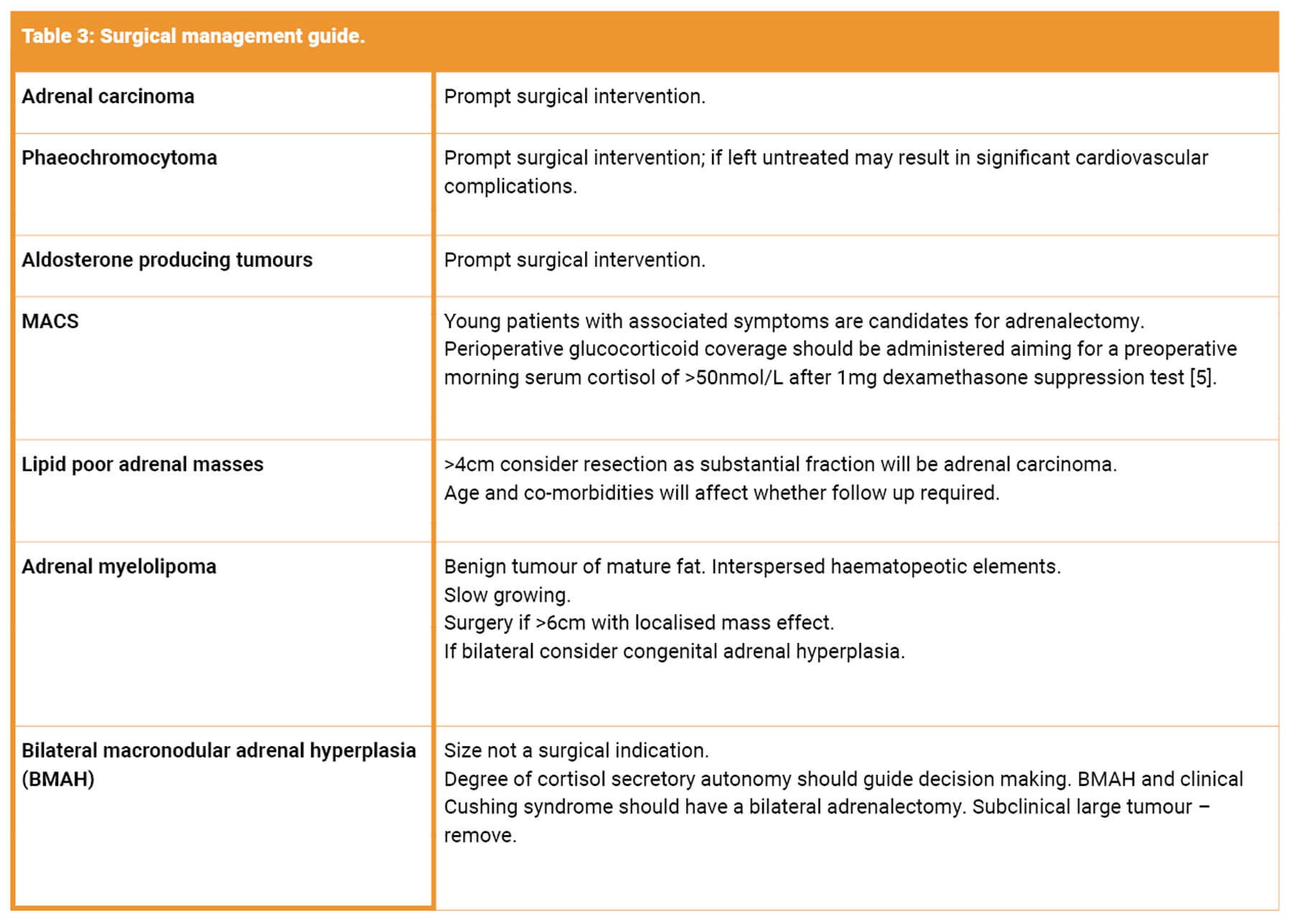
An incidentaloma refers to an adrenal lesion >1cm discovered incidentally during radiologic examination. Identifying a malignant and / or functioning lesion is critical for management. However, as the majority of lesions are benign, the challenge is the identification of malignant or hyperfunctioning lesions whilst sparing patients with benign lesions unnecessary investigations and treatment, and the anxiety and harm associated with that.
Epidemiology
With the use of high-resolution scanners, incidentalomas have been reported on 3–7% of adults, with an increased incidence with age [1]. Data from autopsies report a prevalence at 2%, likely higher in obese, diabetic, hypertensive individuals. Incidentalomas may be bilateral in approximately 15% and may be associated with a number of different pathologies [2].
Differential diagnosis
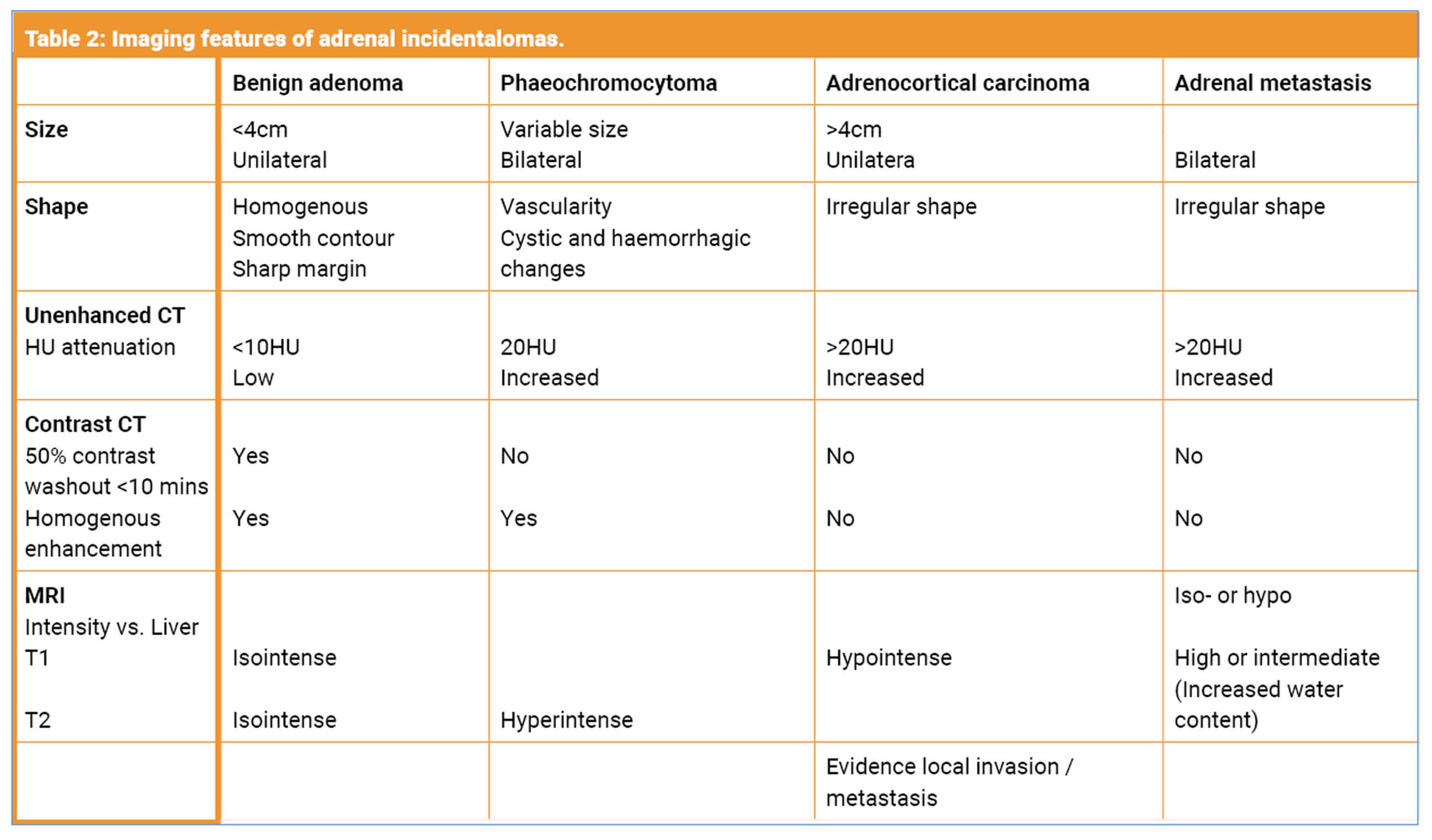
A benign, non-hyperfunctioning adenoma is the most common type of adrenal mass. However, the adrenal gland is a common site for metastasis from other cancers. Less commonly incidentalomas may be primary adrenal tumours such as phaeochromocytomas, aldosteronomas and adrenal cortical carcinomas. Approximately 14% are functioning tumours that secrete cortisol, catecholamines, and rarely, androgens [1].
Benign lesions
The identification of a benign adrenal mass relies on the imaging characteristics and functional assessment. Benign adenomas are lipid rich when compared to carcinomas. Lesions such as myelolipomas have the presence of macroscopic fat and therefore lower attenuation on CT (≤10 HU). Cysts or haemorrhage also have a low attenuation and can be differentiated on imaging if fluid is present [2]. Other benign pathology includes calcified lesions such as old haematoma or calcification of previous granulomatous infection or nodular hyperplasia [1].
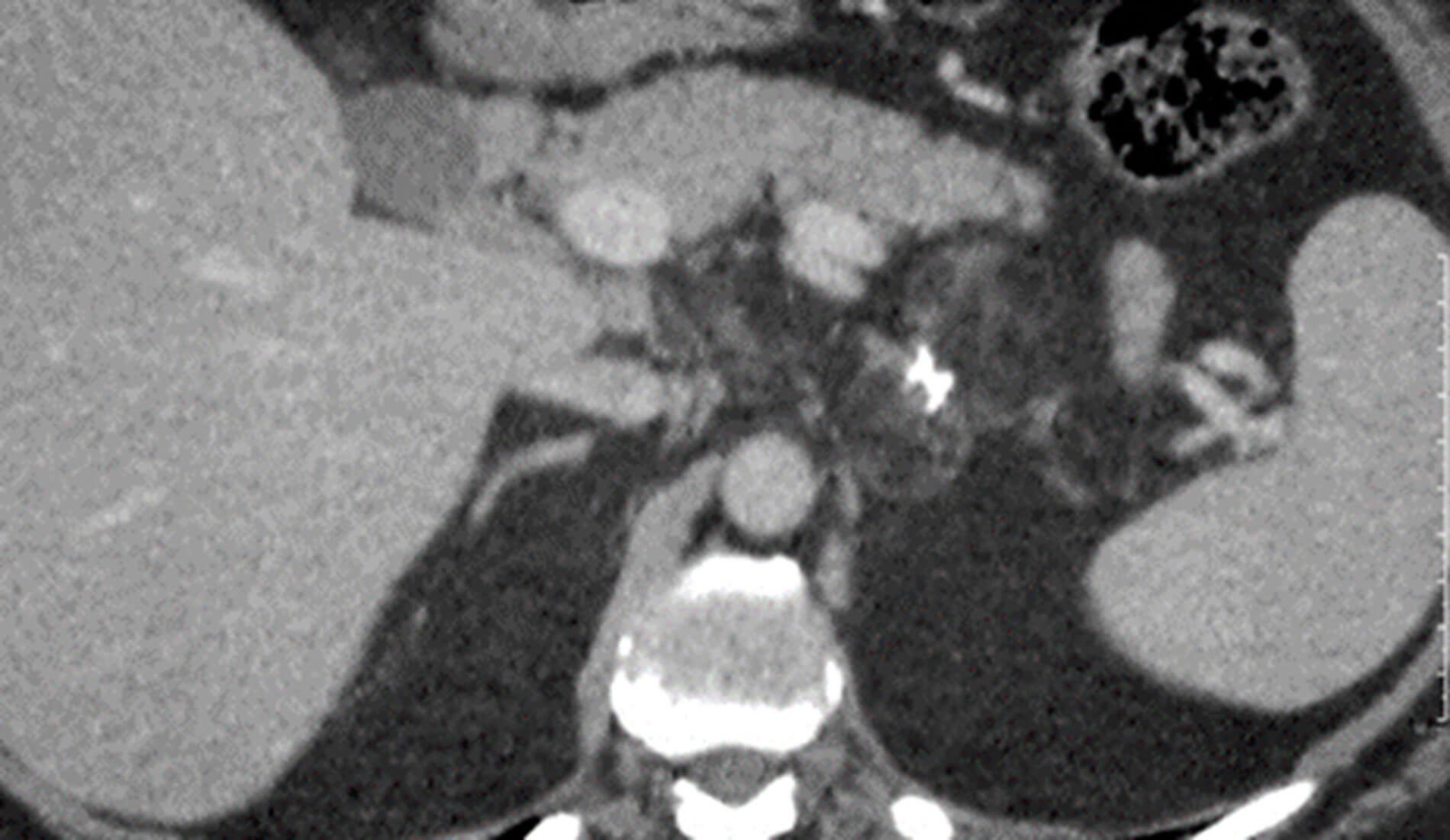
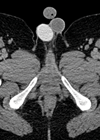
Figure 1: Axial portal venous CT image shows a 5.5 cm bilobed low attenuation mass arising from the left adrenal gland. There are foci of coarse calcification centrally and a large macroscopic fat content consistent with a benign myelolipoma.
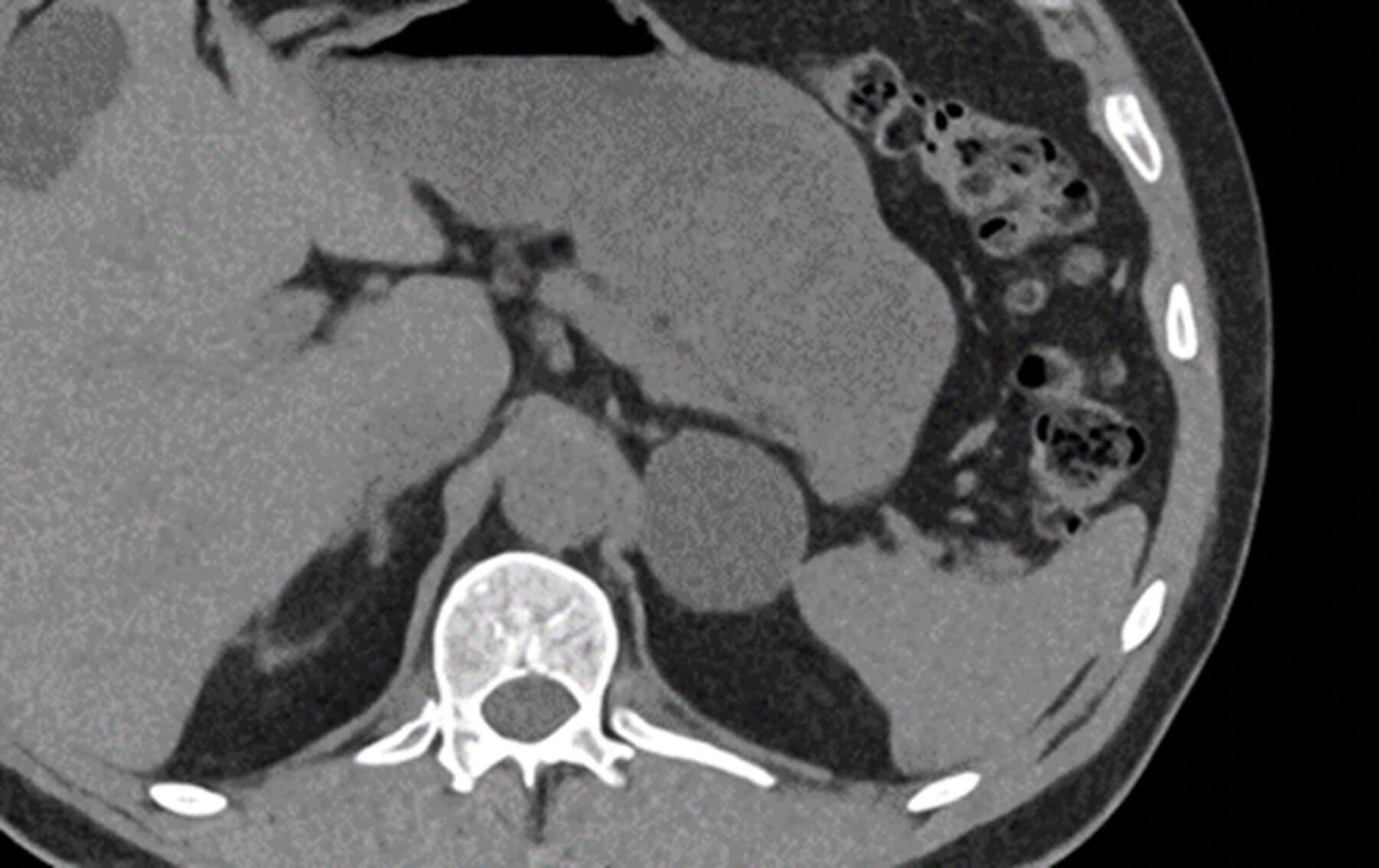
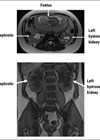
Figure 2: Axial non contrast CT. The left adrenal gland shows a well circumscribed uniform low attenuation mass (CT density -2HU) consistent with a benign lipid rich adrenal adenoma.
Malignant lesions
Adrenal cortical carcinoma (ACC)
These lesions are rare, with an incidence of 0.7–2 per one million, with a bimodal age distribution with peaks in children and in the fourth and fifth decade of life [3,4]. Most are sporadic but may be associated with hereditary cancer syndromes such as Li-Fraumeni, Beckwith-Widemann or multiple endocrine neoplasia Type 1 [3].
Symptoms of hormone excess occur in 60% of cases of ACC, with 45% of these having Cushing syndrome or mixed Cushing and virilisation syndrome (combination of glucocorticoid and androgen excess) [3].
In tumours producing glucocorticoid the symptoms of weakness, central obesity and insomnia can develop over three to six months. Non-functioning ACC may produce symptoms due to local tumour growth, resulting in flank and abdominal pain [3].
Adrenal metastasis
Adrenals are a common site for metastases, commonly from melanoma, lung, breast, gastrointestinal, kidney, pancreas, thyroid cancer and prostate. Adrenals are the fourth most common site of metastasis after lungs, liver and bone. An adrenal incidentaloma may pose a diagnostic challenge in patients with known previous malignancies, as over 50% are metastatic lesions but the rest are not.
Hyperfunctioning tumours
Approximately 14% are hyperfunctional. Investigation is essential as cortisol and aldosterone secretion are associated with increased risk of cardiometabolic disease and cardiovascular morbidity and mortality [1].
Phaeochromocytoma
Accounting for 5% of incidentalomas, phaeochromocytomas arise from the chromaffin cells of the adrenal medulla and secrete catecholamines (dopamine, noradrenaline and / or adrenaline), with a peak incidence in the fourth and fifth decade of life and affecting males and females equally. Pheochromocytomas may originate outside the adrenal gland, known as paragangliomas. Known genetic associations include von Hippel-Lindau syndrome, multiple endocrine neoplasia Type 2 and neurofibromatosis Type 1 [2].
Symptoms of the classic triad of sweating, headache and tachycardia are found in 50% of patients. The most common sign is paroxysmal hypertension which is present in half of this population, the remainder demonstrate persistent hypertension or a minority are normotensive [2,3].
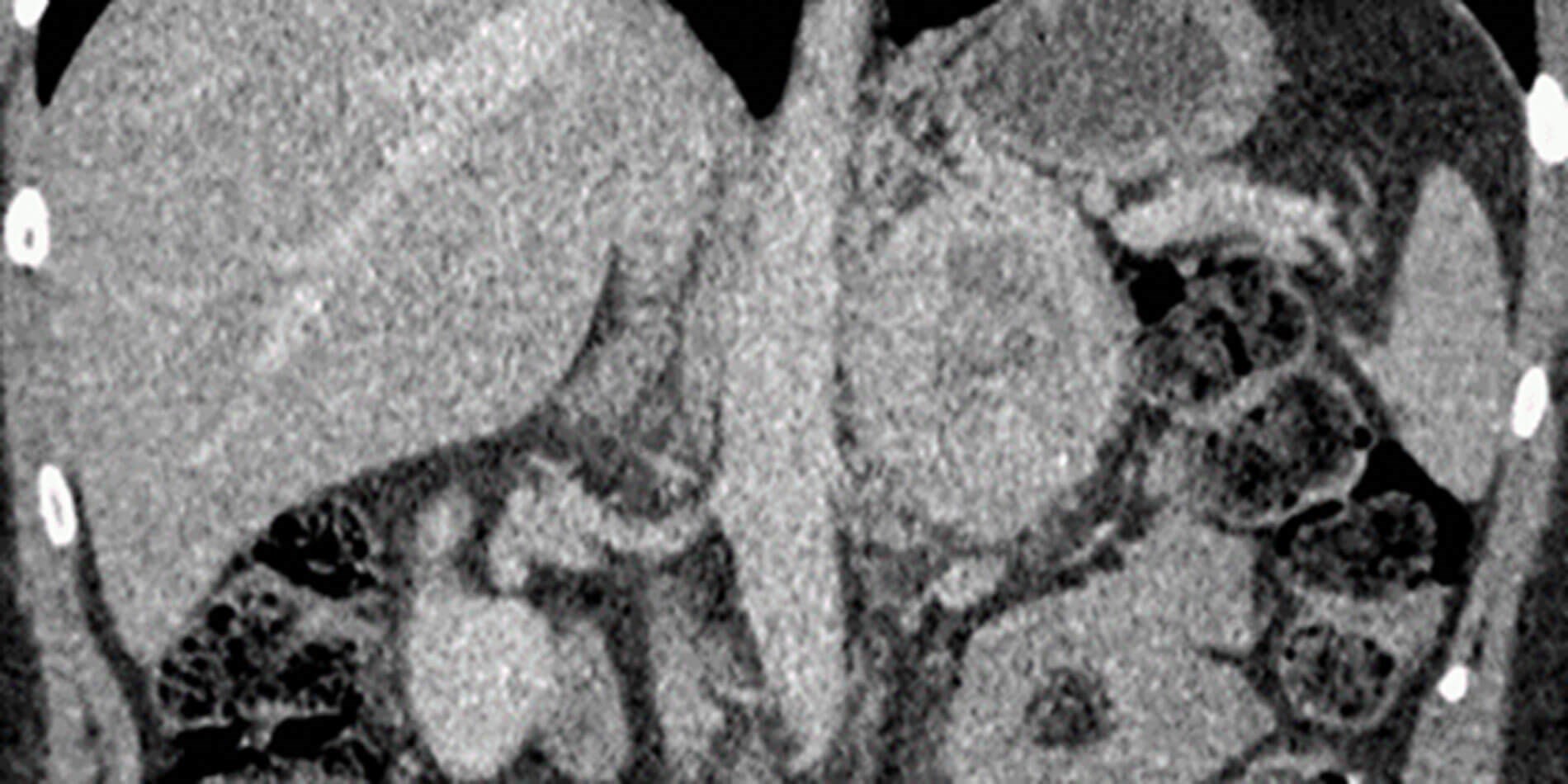
Figure 3: Post contrast coronal CT shows a large mass showing avid enhancement in the peripheral portions and a central non enhancing necrotic area.
Other functioning adenomas (e.g. aldosteronomas)
These make up less than <1% of incidentalomas. In aldosteronomas the excess aldosterone secretion is independent of the renin-angiotensin system. The majority of patients have hypertension and a small number will be have hypokalaemia.
The diagnostic dilemma: is this lesion malignant?
A. Imaging characteristics
To identify whether a mass is benign or malignant a dedicated CT adrenal protocol allows both density measurement and assessment of contrast washout. Reviewing previous imaging allows assessment of the rate of growth.
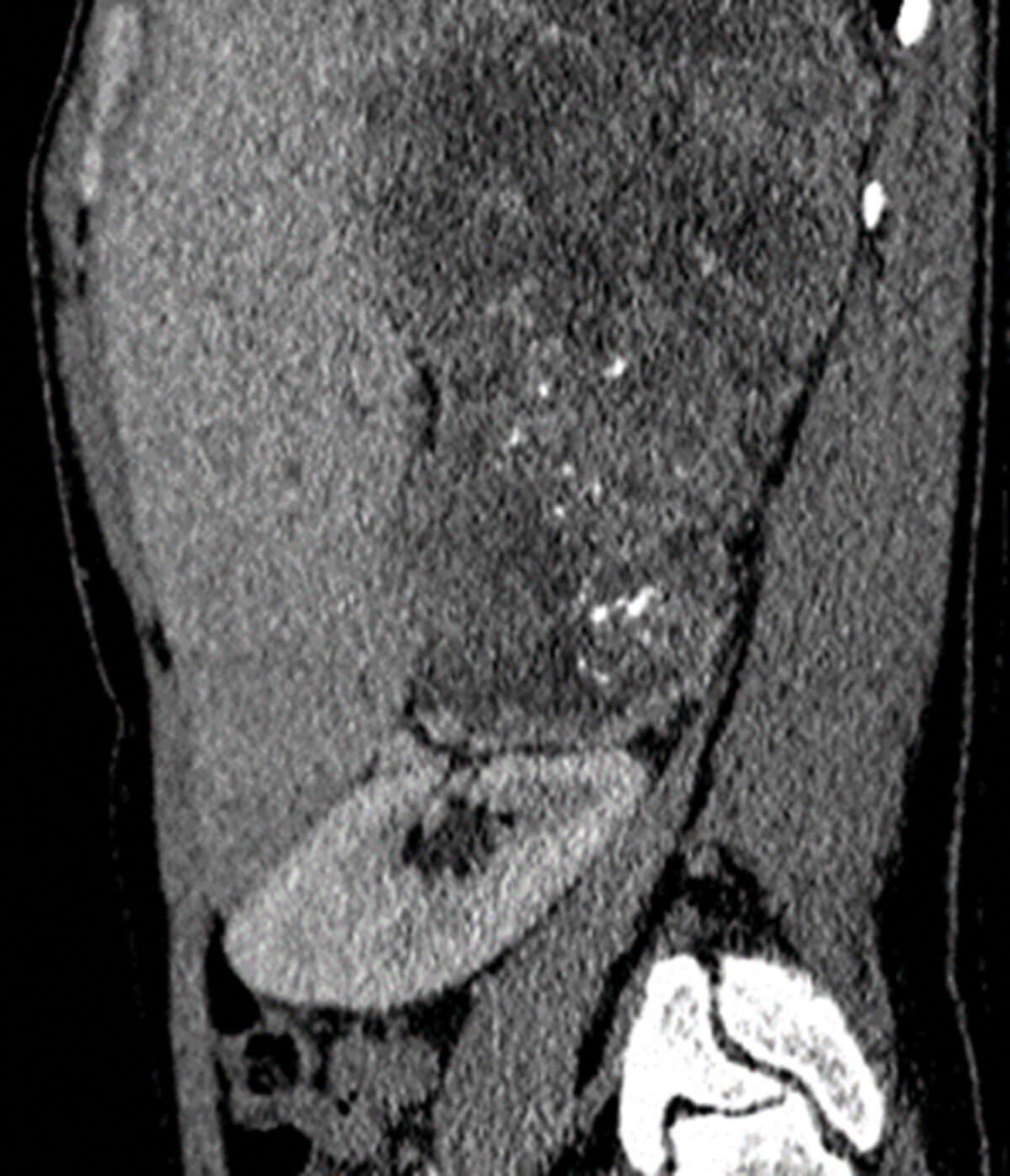
Figure 4: Sagittal post contrast CT showing a large and heterogenous mass arising from the right adrenal gland proven to be adrenocortical carcinoma on histology.
Specific characteristics of the lesion will help identify if a lesion is benign or malignant:
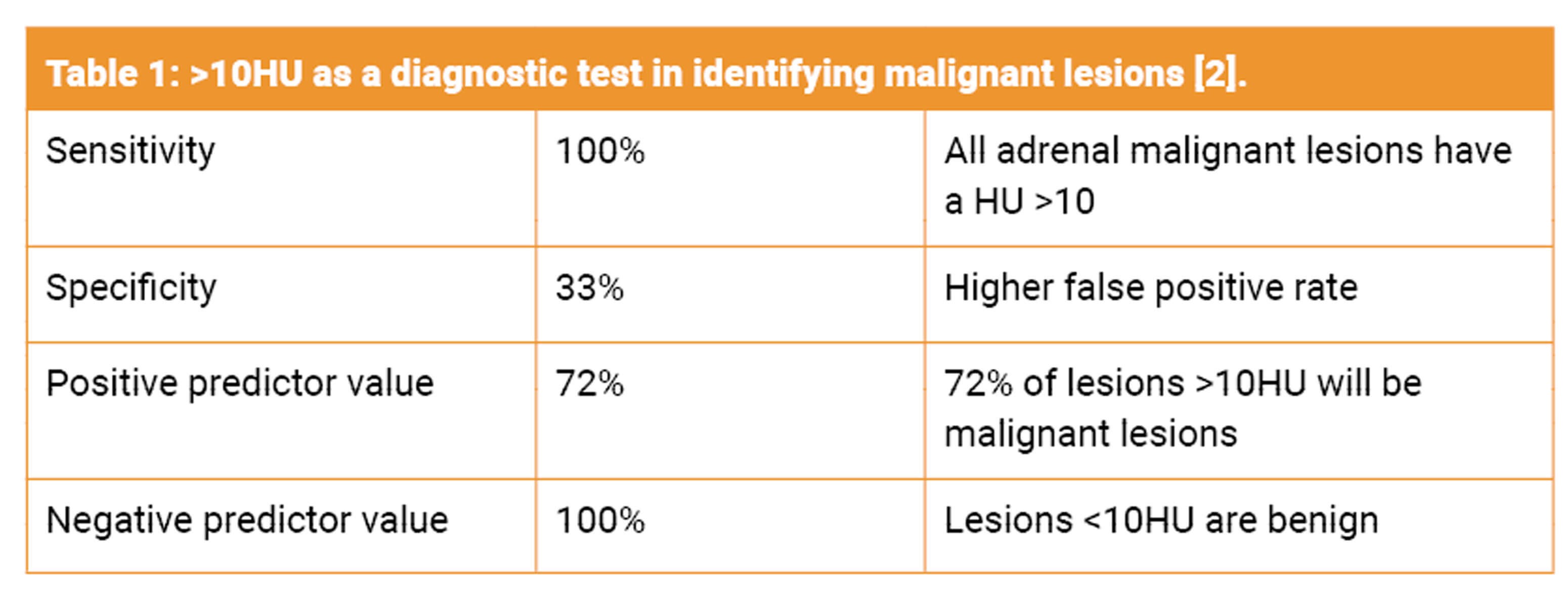
- Size: 90% of malignant incidentalomas are >4cm and very heterogenous. Studies have shown that having a 4cm cut off has a 93% sensitivity for malignancy, although with low specificity (76% masses over 4cm were benign) [2]. Surgical removal should be considered for lesions over 4cm to avoid missing adrenal carcinoma, especially in a younger age group. Smaller tumours lend themselves to curative resection.
- Unenhanced attenuation: Lesions <10HU with a smooth border and homogenous features are likely to be a benign adenoma. The CT attenuation of adipose tissue is between -20 and -150, and as adenomas have a higher fat content they tend to have a low attenuation. Normal kidney tissue is between 20-150HU. A non-adenoma has a higher attenuation. Unfortunately, 30% of benign adenomas may only contain a small amount of lipid [2]. Malignant lesions have an unenhanced attenuation of >10HU.
- Delayed contrast CT: If 50% of contrast washout occurs after 10 minutes it is likely a benign adenoma [2].
MRI can be utilised for follow up, reducing exposure to ionised radiation. It is second line in diagnosis except in the young patient, pregnant women or those with contrast allergy. For diagnostic purposes MRI with T1 and T2 weighted imaging can distinguish between malignant and benign pathology. In MRI chemical shift imaging the resonant frequency of protons in tissue can be used to identify the type and amount of tissue. This allows radiologists to identify the presence and amount of intra cytoplasmic fat, with a higher fat content indicating a benign adenoma [4].
18F-FDG PET/CT
Fluorine-18-fluorodeoxyglucose (18F-FDG) can be used to evaluate indeterminate masses. This imaging modality can assess for disease beyond the adrenal gland. False negatives can occur due to the small size of tumours, low avidity, necrosis or prior chemotherapy. False positives can occur due to increased metabolic activity in benign lesions, such a phaeochromocytomas and adrenal adenomas [1].
123I-MIBG SPECT CT
Iodine-123-labeled metaiodobenzylguanidine (123I-MIBG) SPECT CT is useful in staging phaeochromocytomas as well as assessing extra adrenal disease and for postoperative assessment [1].
11C-metomidate PET/CT
An alternative PET/CT to lateralise a primary aldosteronoma when adrenal venous sampling is not possible or where results have been inconclusive [1].
Other statistically significant predictors of malignant incidentalomas included older age and male sex.
B. The role of biopsy
The EAU guidelines only advise biopsy if three criteria are fulfilled:
- The lesion is hormone inactive (phaeochromocytoma should be excluded as biopsy or fine needle aspiration risks haemorrhage and hypertensive crisis).
- Lesion has not been conclusively characterised as benign on imaging.
- Clinical management of patient would be altered by the histology [5].
The diagnostic dilemma: is this lesion functional?
Primary aldosteronism (PA)
This is the autonomous excess secretion of aldosterone, independent of the renin-angiotensin system. It results in decreased plasma renin. Hypertension is a key manifestation, associated with 1-2% of mild hypertension and 20% of resistant hypertension. Hypokalaemia is a late presentation, with over half of patients having a normal potassium level [1]. A patient with an adrenal mass with hypertension or unexplained hypokalaemia should have the aldosterone / renin ratio measured. If elevated, management depends on whether the lesion is unilateral or bilateral. Unilateral lesions (either adenoma or hyperplasia) are treated surgically. Bilateral lesions are managed with lifelong minerocorticoid receptor agonists. Adrenal venous sampling should be considered for lateralisation unless the patient is <40-years old or with a clear unilateral >1cm lesion. PA carries an excess cardiovascular and renal risk mediated by both hypertension and aldosterone’s direct effect on the tissue of target organs [1].
Autonomous cortisol secretion (mild autonomous Cushing’s syndrome)
The most common hormonal abnormality associated with the adrenal adenomas is autonomous cortisol secretion. In patients with increased cortisol it is important to rule out a phaeochromocytoma, which is more likely if the lesion is >10HU on unenhanced CT [1,2].
Clinically the symptoms and signs may be less exaggerated than in Cushing’s, but the excess cortisol secretion may result in hypertension, diabetes, weight gain, hyperlipidaemia, osteoporosis and atherosclerosis [2].
Initial work-up involves a dexamethasone suppression test and analysis of plasma metanephrines. If the plasma metanephrines are equivocal, 24-hour urine collection for metanephrines can be undertaken. Serum cortisol concentration of ≤50nmol/L is the diagnostic threshold to exclude autonomous cortisol secretion [1]. Cortisol 51-138nmol/L with no Cushing’s signs indicates possible mild autonomous cortisol secretion (MACS) [1].
High levels of cortisol due to a hyperfunctioning adenoma will result in a low plasma adrenocorticotrophin level (ACTH.) ACTH testing following overnight dexamethasone suppression may allow confirmation of MACS with ACTH measurements of <2pmol/L1.
Catecholamine excess
This presents with a triad of headaches, palpitations and profuse sweating and paroxysmal hypertension. Initial testing is with plasma free or total fractioned urinary metanephrines.
Local management for endocrinology work-up
At Arrowe Park hospital the endocrinology work-up for incidentalomas is:
- 24-hour urine free cortisol and catecholamines (can both be done in one 24-hour collection).
- Renin / aldosterone blood test only if the patient has hypertension and / or hypokalaemia.
Surgical approach
A minimally invasive approach is recommended for a benign adrenal mass causing hormone excess. Open surgery should be considered if the adrenal mass displays features suggestive of local invasion, and should be undertaken in a high-volume unit [5]. For pheochromocytomas, aldosteronomas, cortisol-secreting tumours and adrenal incidentalomas surgery can be done laparoscopically via transperitoneal or retroperitoneal approaches or as open surgery.
Monitoring when surgery is not required
For benign incidentalomas many units have their own management pathways. We present our own algorithm at Arrowe Park Hospital which includes a protocol for repeat imaging at 12 months depending on the size of the lesion and appearance. If the lesion increases in size >1cm in the follow up period, surgery should be considered.
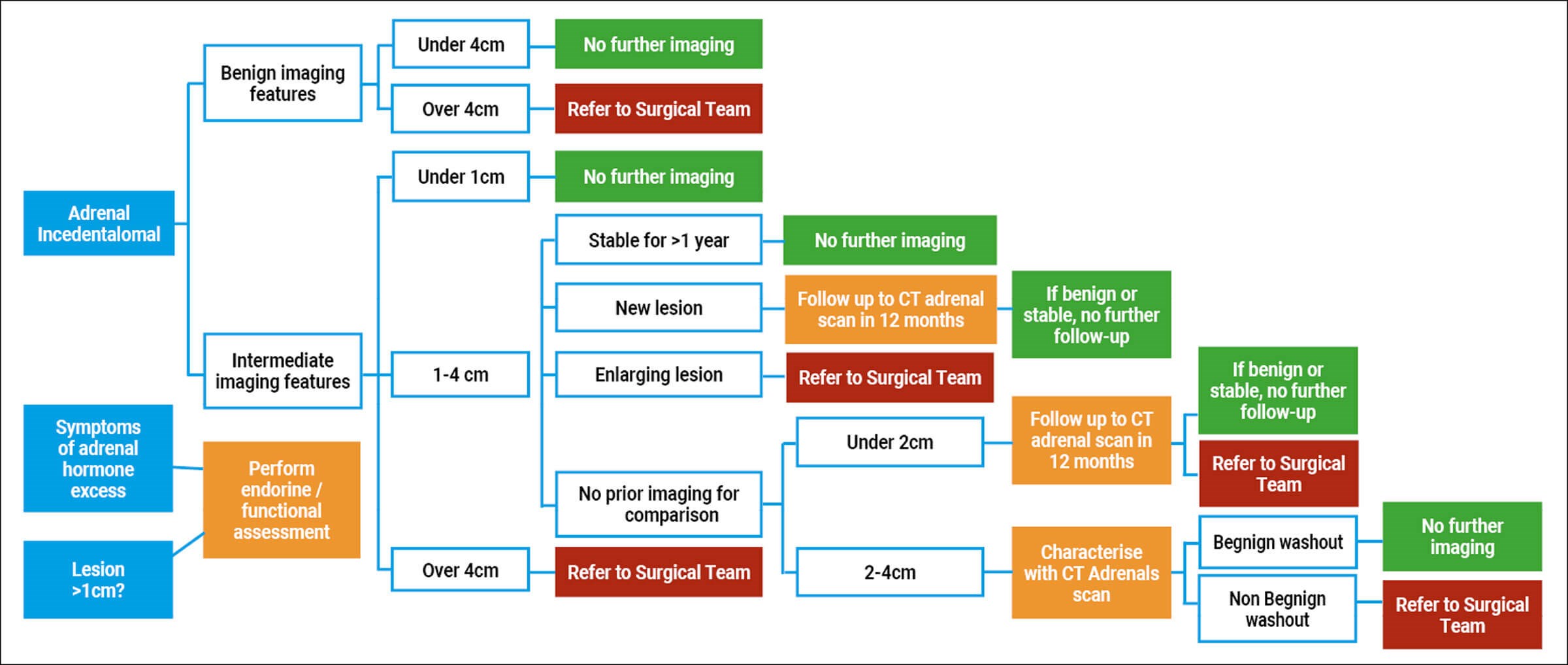
Figure 5: Arrowe Park diagnostic and treatment pathway. Courtesy of Dr S Amin et al: Management of Incidental Adrenal lesions detected on Imaging. 2021.
References
1. Cuthbertson DJ, Alam U, Davison AS, et al. Investigation and assessment of adrenal incidentalomas. Clin Med (Lond) 2023;23(2):135-40.
2. Young WF, Kebebew E. Evaluation and management of adrenal incidentaloma. Up To Date
https://www.uptodate.com/contents/
evaluation-and-management-of-the-
adrenal-incidentaloma?search=Evaluation
%20and%20management%20of%20adrenal
%20incidentaloma&source=search_result&selected
Title=1~24&usage_type=default&display_rank=1
[accessed 5 December 2023].
3. Lacroix A. Clinical presentation and evaluation of adrenocortical tumours. Up To Date
https://www.uptodate.com/contents/clinical-presentation
-and-evaluation-of-adrenocortical-tumors?
search=Clinical%20presentation%
20and%20evaluation%20of%20adrenocortical
%20tumours.%20&source=search_result&selected
Title=1~150&usage_type=default&display_rank=1
[accessed 5 December 2023].
4. Adam SZ, Nikolaidis P, Horowitz JM, et al. Chemical shift MR imaging of the adrenal gland: principles, pitfalls and applications. Radiographics 2016;36(2):414-32.
5. Fassnacht M, Tsagarakis S, Terzolo M, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumours. Eur J Endocrinol 2023;189(1):G1-G42.
Declaration of competing interests: None declared.