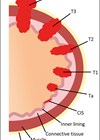
Bladder malignancy is one of the commonest malignancies of the renal tract, accounting for approximately 6% of male malignancy and 2% of female malignancy. The incidence increases with patient age with 70% of patients being over the age of 65 years at presentation. Bladder carcinoma is three to four times more common in men than women and has a high recurrence rate.
Ninety percent of bladder carcinomas are transitional cell carcinomas (TCC), and most commonly involve the lateral bladder walls and trigone. Other subtypes include squamous cell, sarcomatoid and lymphoepithelioma-like tumours. Adenocarcinoma is rare and is associated with a patent urachus and urachal tumour.
Eighty to eighty-five percent of urothelial tumours are non-muscle invasive and confined to the mucosa or submucosa, and generally have a good prognosis. Twenty to twenty-five percent of bladder malignancies are muscle invasive [1], and nearly all cases of squamous and adenocarcinoma are invasive at the time of diagnosis. Thirty-three percent of patients have multifocal disease at presentation and have an increased risk of invasive and recurrent disease.
There are several known risk factors, including genetic predisposition, smoking, occupational exposure, exposure to ionising radiation and previous schistosomiasis.
Patients present with painless haematuria in 80% cases, but may also present with dysuria, urinary frequency, and urinary urgency.
Ultrasound
Ultrasound can identify a primary bladder tumour, but will not clearly see tumours that are flat or small. If the bladder is not adequately distended at the time of scanning, tumours are likely to be missed as the bladder wall can look concentrically thickened. The main role of ultrasound is to exclude hydronephrosis or a renal cause for haematuria.
Hydronephrosis is an independent predictor of advanced stage bladder cancer and patients who present with hydronephrosis have a worse clinical outcome [2]. It is reported to predict extra-vesical and node positive disease.
Computed tomography
The accuracy of CT for local staging of bladder malignancy is only 40-60%, but it has a high sensitivity and specificity of over 90% for the diagnosis of bladder malignancy in patients with haematuria [3]. It is usually performed to assess the upper tracts as synchronous upper tract tumours are seen in 2.5% patients with bladder malignancy, and following cystectomy an upper tract TCC is seen in 1.8-6% cases.
Images should be obtained that allow full assessment of the urothelial system and assessed for any urothelial thickening, filling defect or irregularity. As with ultrasound, small flat bladder tumours may not be appreciated on CT, particularly if the bladder is not adequately distended. Tumours at the bladder base can also be difficult to see, particularly if the patient has an enlarged prostate.
CT also allows assessment of any nodal or metastatic disease, evaluation of the distal ureters and identification of any anatomical variants, such as duplex kidney and ureteric insertion, which should be identified prior to cystectomy.
CT is the main imaging modality for follow-up and should include assessment of the upper tracts to assess for an upper tract TCC. There is currently no agreed consensus about the frequency or timing of CT follow-up in patients with bladder malignancy.
Magnetic resonance imaging
Accuracy of MRI for staging of the primary tumour is reported at 73-96% with mean value 85%. The staging accuracy values are 10-33% higher than CT, and MRI is considered superior to CT due to its high soft-tissue contrast resolution and multiplanar imaging capabilities [4]. MRI is superior to CT in demonstrating invasion of the bladder wall and being able to differentiate T2a and T2b disease.
Advances in MRI now enable multiparametric imaging to be performed, which includes a combination of anatomical and functional sequences, and is likely to further improve the staging accuracy [5]. The accuracy of contrast enhanced MRI in determining bladder tumour stage is reported at 52-93%, and when used in conjunction with diffusion weighted imaging and T2 sequences, the accuracy has been reported as 92%.
Multiparametric MRI uses a combination of T1 and T2 images as well as dynamic contrast enhanced sequences and diffusion weighted imaging (DWI). High spatial resolution is achieved using a surface phased-array coil, thin sections (<3mm) and a large matrix size. Sequences are acquired in different planes and assessed both independently and in conjunction with each other.
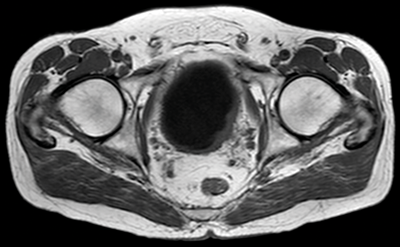
Figure 1. T1 MRI showing left lateral bladder tumour seen as intermediate signal compared with low signal urine in the bladder. There is fat stranding adjacent to the tumour, in keeping with early T3b disease.
T1 sequences are useful for identifying the primary tumour, as it can be seen against the dark urine in the bladder (Figure 1). T1 sequences are valuable for assessing perivesical fat infiltration as the fat adjacent to the bladder is of high signal. T1 sequences are also useful for assessment of nodal disease or bone metastases.
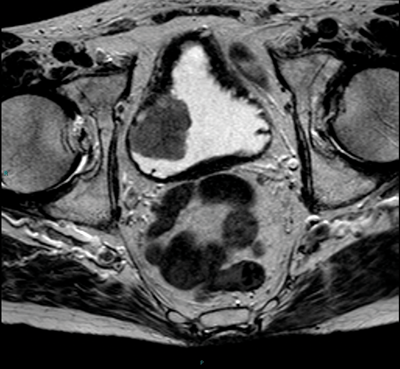
Figure 2. Axial T2 MRI showing a right sided tumour with the no evidence of extra-vesical extension.
T2 images should be obtained or reconstructed in three planes. T2 images allow assessment of the primary tumour and the bladder wall (Figure 2), and are superior to T1 images at assessing for invasion into adjacent organs such as the prostate or uterus. As well as axial imaging, a lateral bladder tumour or tumour at the dome is best assessed on coronal imaging, and an anterior tumour in the sagittal plane.

Figure 3a. DWI image in the same patient as Figure 2 shows the tumour to be high signal.
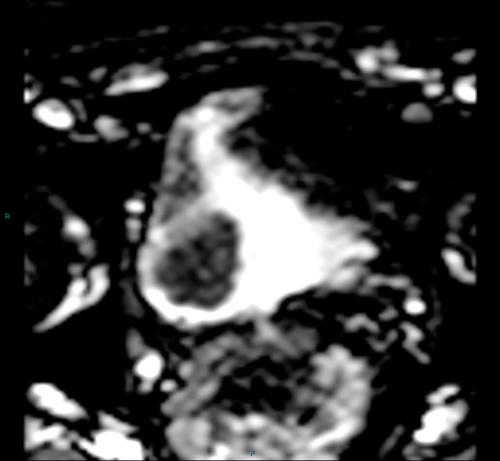
Figure 3b. ADC map shows restricted diffusion within the tumour.
Diffusion weighted imaging relies on proton diffusion properties in water. Diffusion images are obtained using multiple B-values, and the tumour will be high signal on the high B-value image and then show low signal on the corresponding apparent diffusion coefficient (ADC) map (Figure 3a and 3b). The ADC map reflects movement of water molecules and represents capillary perfusion and diffusion. Images are assessed in conjunction with the T2 images at the same level.
Dynamic contrast enhanced T1 images allow assessment of blood flow within a tumour as the tumour takes up more contrast than the remainder of the bladder wall. The normal bladder wall does not enhance avidly on very early contrast enhanced images compared with a tumour due to neovascularisation. Studies have been undertaken looking at dynamic contrast enhanced images and tumour has been shown to enhance four seconds earlier than inflammatory tissue following a biopsy or resection [6].
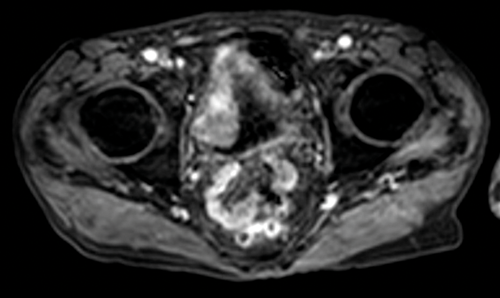
Figure 4. Early contrast enhanced images of the same patient as Figures 2
and 3 show avid enhancement of the internal half of the bladder wall.
If the tumour is seen to enhance, close evaluation is needed to assess if this is full thickness or less than half the thickness of the wall in order to provide radiological staging (Figure 4). The distal ureter may also be seen to enhance if the tumour is close to the ureteric orifice (Figure 5).
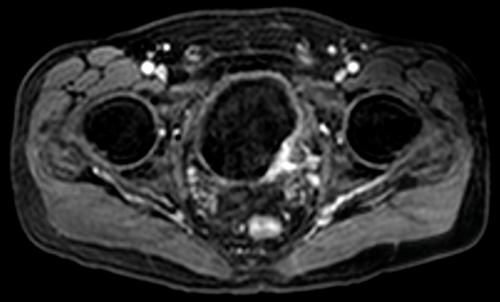
Figure 5. Contrast enhanced image of the same patient as Figure 1 shows full
thickness enhancement of the bladder wall and enhancement of the distal left ureter.
CT and MRI are unable to diagnose T3a disease, but can detect T3b disease if tumour is seen extending outside the bladder wall. The adjacent fat should be assessed to look for fat stranding and extra-vesical disease. If imaging is performed following resection, there may be fat stranding, due to surrounding oedema or inflammation, so this should be treated with caution and closely evaluated on post-contrast imaging.
The pelvis and abdomen should be assessed for the presence of lymph nodes. It is often most useful to do this on the coronal view as more of the pelvic lymph nodes are included in this plane and can be easier to visualise (Figure 6).
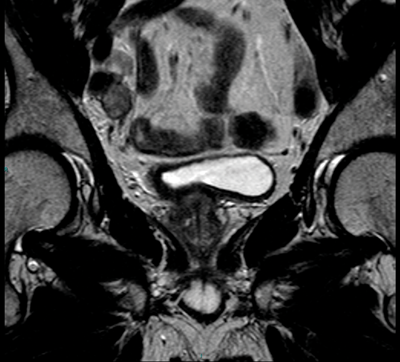
Figure 6. Coronal T2 image shows right iliac nodes adjacent to the iliac vessels.
The ureters should be assessed where possible, but often they are collapsed on MRI and therefore evaluation is difficult. CTU is more sensitive to exclude an upper tract tumour, and should be performed in conjunction with MRI.
MRI sequences should include imaging of the upper abdomen to assess for hydronephrosis, metastatic disease and any upper abdominal lymphadenopathy.
Conclusion
MRI is most useful for local staging of a bladder tumour. CT is used for distant staging, assessment of the upper tracts and follow-up of patients with previous bladder malignancy.
References
1. Verma S, Rajesh A, Prasad SR, et al. Urinary bladder cancer: Role of MR Imaging. Radiographics 2012;32:371-87.
2. Luo HL, Kang CH, Chen YT, et al. Severity of hydronephrosis correlates with tumour invasiveness and urinary bladder recurrence of ureteric cancer. BJU Int 2013;112:489-94.
3. Caterina M, Giunta S, Finocchi V, et al. Primary cancer of the urinary bladder: CT evaluation of the T parameter with different techniques. Abdom Imaging 2001;26(4):433-8.
4. Tekes A, Kamel I, Imam K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR 2005;184:121-7.
5. De Hass, RJ, Steyvers MJ, Futterer JJ. Multiparametric MRI of the Bladder: Ready for Clinical Routine? American Journal of Roentgenology 2014;202(6):1187-95.
6. Barentsz JO, Jager GJ, van Vierzen PB, et al. Staging urinary bladder cancer after transurethral biopsy: value of fast dynamic contrast enhanced MR imaging. Radiology 1996;201:185-93.
IMAGING TIPS
-
Avoid imaging with an empty bladder as it is easier to see the tumour next to urine in the bladder.
-
The dome of the bladder is better seen on reformatted coronal and sagittal planes rather than the conventional axial plane.
-
Careful evaluation of the distal ureters should be performed if the tumour involves the ureteric orifices.