This article takes a look back over recent years at new innovations and developments relating to muscle-invasive bladder cancer (MIBC) specifically, and will also touch upon what the future may hold.
This article is also written as a continuation of sorts from a previous written article on non-muscle invasive bladder cancer, and reference will be made to that previous piece (Saunders H, Cresswell J. Recent developments in bladder cancer – NMIBC. Urology News 2022;26(4):15-18).
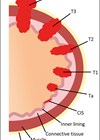
Of the 10,300 individuals diagnosed with bladder cancer every year in the UK [1], 25% of these patients will have disease that extends beyond the mucosa (T1) [2], invading the muscle beneath (classified as T2) or beyond (T3 or T4), as shown in Figure 1.
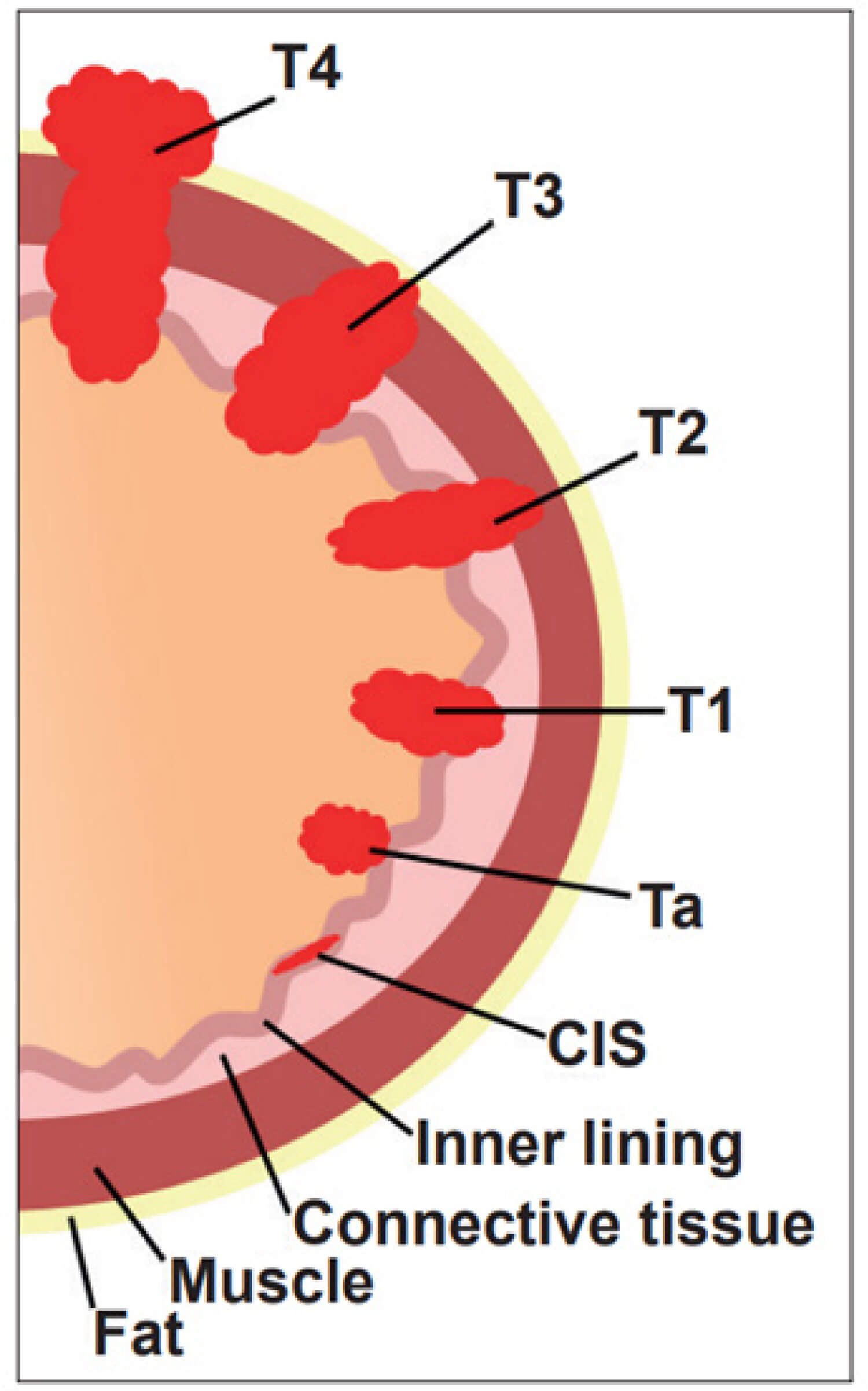
Figure 1: Bladder cancer staging.
The detection and diagnosis of bladder cancer was discussed more in depth in the previous article reviewing NMIBC developments. However, it is worth revisiting the use of MRI and the vesical imaging-reporting and data system (VI-RADS) with regards to MIBC.
MRI and VI-RADS
The European Association of Urology (EAU) is placing greater value on the use of MRI, changing their guideline from the previous year to deem MRI as superior to CT in terms of differentiating T1 from T2 disease [2]. The introduction of the VI-RADS scoring system in recent years has further strengthened the usefulness of MRI (specifically multiparametric (mp)MRI), giving suspicious lesions a score based on the likelihood of muscle invasion, which may help expedite radical treatment in patients with NMIBC [3].
For MIBC, an exciting recent development is the potential that mpMRI could be used as a first-line test to locally stage bladder cancer as opposed to transurethral resection of bladder tumour (TURBT). Early findings from the BladderPath study have posited that this may indeed be a feasible option for the future [4].
In recent years there has also been inquiry into the ability of MRI scanning to predict and assess response to medical therapies, in a bid to allow for better patient selection for bladder-sparing approaches. Diffusion-weighted (DW) MRI can express the diffusion of water molecules as a value which allows for differentiation of normal and abnormal (malignant) tissue [5]. This feature has been taken one step further in patients with MIBC, as it has been used to accurately predict which patients will have a favourable response to chemotherapy prior to radical treatment [5]. This information could be used to aid in the decision-making of opting for cystectomy or bladder-sparing treatment.
Continuing along the same vein of utilising MRI to predict therapy response, an offshoot of the PURE-01 study (examining disease remission post-cystectomy with the administration of pembrolizumab preoperatively) evaluated whether mpMRI could be used to identify patients that would have a strong, favourable response to checkpoint inhibitors [6,7]. Whilst there were some limitations to this offshoot study, the results were promising and represents the first step towards identifying patients with MIBC that will respond to a single-agent checkpoint inhibitor [7]. Yet again, this may provide the means of predicting which patients will be able to proceed along the bladder-sparing path.
Mention has been made to the various medical treatments and bladder-sparing options for patients with MIBC, so it is now worth delving into these options to see how they have changed over the years.
The (neo)adjuvant setting
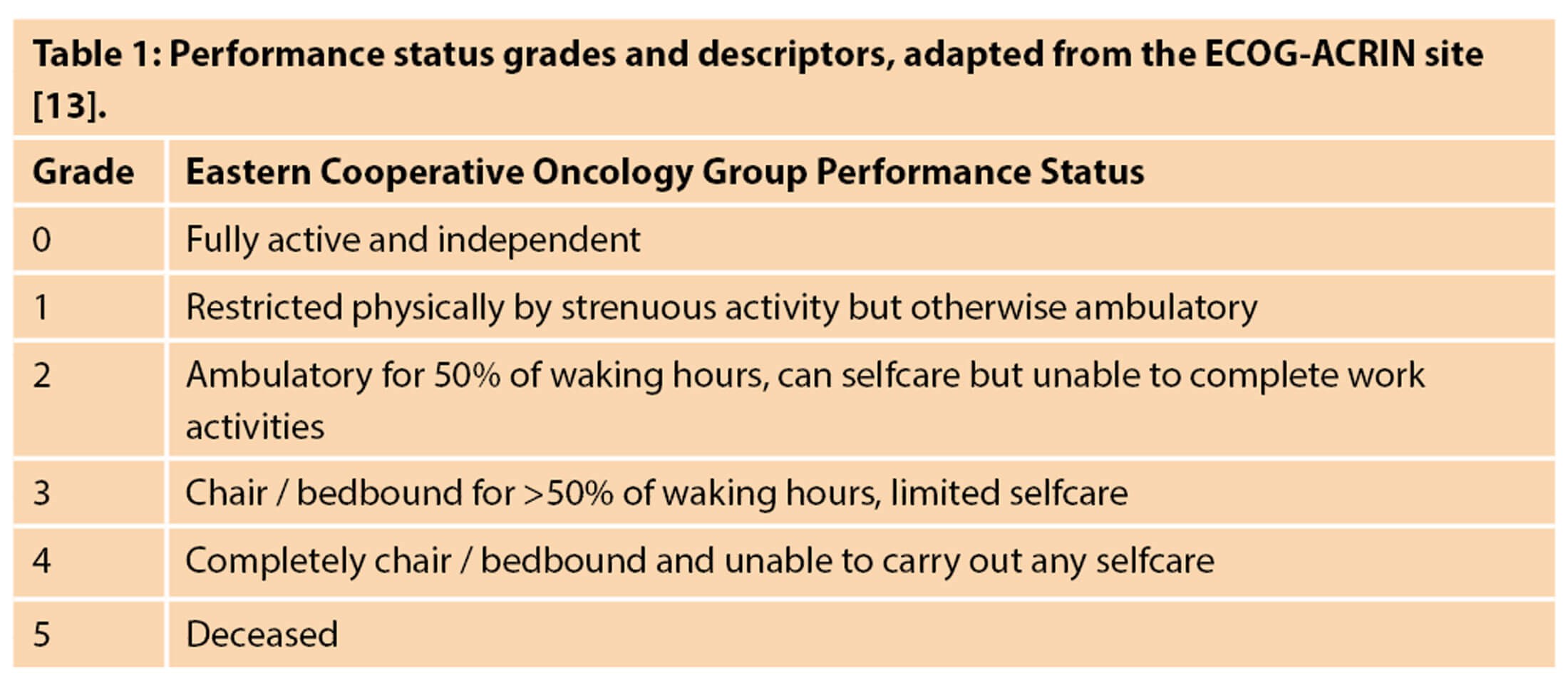
There are a number of different treatment pathways and algorithms outlining the management of patients with MIBC, with a separate route for situations when metastasis is present or when there are pathological lymph nodes. The EAU created a pathway showing the typical steps to managing a patient with at least T2 disease without any pathological lymph nodes nor metastasis, with acceptable renal function and good performance status, shown in Figure 2. Performance status grading can be found in Table 1. However, in the UK the National Institute for Health & Care Excellence (NICE) offers the use of radical radiotherapy as an alternative to radical cystectomy [8].
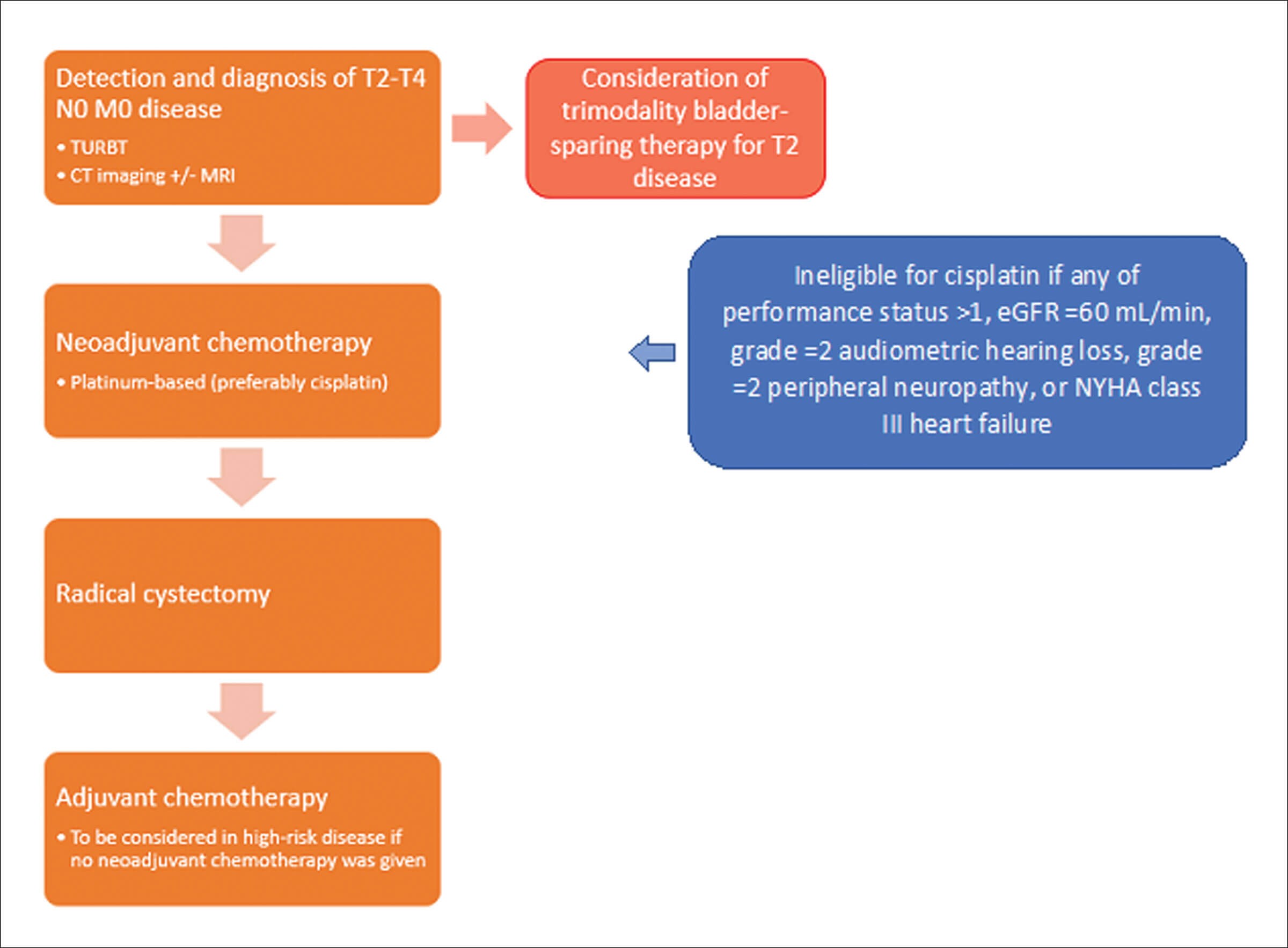
Figure 2: Pathway of the management of T2-T4 N0 M0 disease,
adapted from the EAU 2022 Guidelines on MIBC [2].
Chemotherapy
Cisplatin-based neoadjuvant chemotherapy has been used for decades and as such there has been much research into its effectiveness. Large, randomised controlled trials such as Nordic I and II have been incorporated into a more recent meta-analysis confirming that the use of platinum-based chemotherapy does indeed provide a significant survival benefit [9]. Other developments of these chemotherapy agents over the years have been the introduction of a dose-dense regime, which administers chemotherapy agents over a shorter timeframe with equivocal outcomes and good tolerance [10]. Administering chemotherapy over a shorter timeframe can help alleviate the worry of delaying definitive treatment (i.e. radical cystectomy or radiotherapy).
Another advance has been the introduction of more contemporary chemotherapy agents and regimes, such as gemcitabine-cisplatin. This combination specifically had an improved side-effect profile versus older, more established regimes and so was introduced into the neoadjuvant setting quickly, without the backing of any prospective trials [11]. Gemcitabine-cisplatin has now since been shown to be as effective as other regimes [12]. Following radical cystectomy, the use of adjuvant chemotherapy for higher stage disease remains somewhat in a state of flux, and at present there is not yet enough convincing evidence to confidently recommend the use of adjuvant chemotherapy [2]. In the metastatic disease setting, cisplatin-containing combination therapies remain the preferred treatment option [2].
Immunotherapy
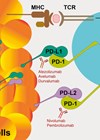
The research and use of immunotherapies is growing and holds some promise for the future. PD-L1 on tumour cells interacts with PD-1 on T-cells to help evade destruction, and drugs such as pembrolizumab and avelumab disrupt this interaction thereby exposing the cancer and allowing for its destruction by the immune system [14], hence they are given the name of checkpoint inhibitors. The efficacy of checkpoint inhibitors has been demonstrated in the metastatic disease setting as either a second-line treatment option or first-line in platinum-ineligible patients [2], but attention is now being turned towards the neoadjuvant scene. The PURE-01 study (mentioned above) recruited patients that had T2-T3b N0 M0 disease and were listed for radical cystectomy.
Prior to their operation, these patients received neoadjuvant pembrolizumab and a staggering 42% of these patients had their disease down-staged to pT0 [15]. Whilst immunotherapy at present is not approved in the neoadjuvant period, there is certainly some promise. Almost akin to the current state of adjuvant chemotherapy, there is conflicting evidence on the efficacy of adjuvant immunotherapy. There have been some positive results with the PD-1 inhibitor nivolumab which demonstrated longer disease-free survival versus placebo among patients with PD-L1 expression of ≥1% [16]; but conversely, the IMvigor010 found that the PD-L1 inhibitor atezolizumab did not improve disease-free survival versus the observation group [17].
For patients with metastatic disease, immunotherapy currently holds the position of being a back-up treatment for patients who are ineligible for the preferred cisplatin and carboplatin chemotherapies, or for patients who have had disease progression despite these treatments [2].
New agents
Emerging onto the scene of managing bladder cancer are antibody-drug conjugates. These intelligent medicines consist of an antibody combined with a cytotoxic agent, which means that there can be targeted release of the cytotoxic agent to the tumour in a very precise manner [11]. The first of these drugs to show promise was enfortumab verdotin. The EV-201 study led to the drug’s quick approval by both the Food and Drug Administration and the European Medicines Agency for patients with locally advanced or metastatic disease that had previously received PD-1/PD-L1 inhibitors and platinum-based chemotherapy [18]. Looking at the near future, the EV-302 study is currently investigating how enfortumab and pembrolizumab fare against gemcitabine and platinum-containing chemotherapy regimens as a first-line treatment, with study completion expected late 2023.
A new type of agent is erdafitinib, which is a fibroblast growth factor receptors (FGFR) inhibitor. FGFR3 specifically, coded by the FGFR gene, is a protein with functions that include regulating cell proliferation and angiogenesis, and mutations or fusions of this protein have been linked to increased incidence of malignancy, including bladder cancer [19]. A study looked at the effectiveness of erdafitinib for patients with unresectable / metastatic disease who had previously received chemotherapy with or without immunotherapy and reported that an objective tumour response was seen in 40% of participants [20]. Other FGFR inhibitors are currently being assessed and showing promising signs [21], and all this has led to the EAU recommending for the screening of FGFR3 mutations at the diagnosis of metastatic disease and labelling abnormal FGFR3 variants as the only validated molecular marker to help predict treatment response [2].
Radiotherapy
As mentioned above, whilst the EAU does not have a strong recommendation for radical radiotherapy, NICE considers it a viable alternative to radical cystectomy. Delivering radical radiotherapy encounters the challenge of the bladder being a mobile organ that can shift and change position during treatment. An interesting development has been the advent of image guided radiotherapy and use of cone beam (CB) CT imaging. This allows for the creation of a ‘plan for the day’. This technique creates a catalogue of images and treatment regimens for the patient, so that on the day of treatment the CBCT can be used prior to treatment, to visualise the position of the bladder, and the best plan can be chosen [22]. The RAIDER trial is hoping to use this technique to allow for greater dose escalation of radiotherapy for radical treatment of MIBC with acceptable rates of toxicity in a bid to improve local disease control [23]. Results are currently awaited as patients are being followed-up.
Conclusion
It is clear that a lot has changed over recent years in both muscle and non-muscle invasive bladder cancer. Technology is improving at an impressive pace, enhancing the detection and evaluation of bladder cancers, which in turn is allowing for better streamlining and management of this disease.
The future also holds much promise, with the rise of immunotherapies and emergence of antibody-drug conjugates showing encouraging results, to the discovery of specific gene / protein mutations that can now be specifically targeted by therapies. Over the next few years, we can expect to see further optimisation and tailored treatments for patients suffering with bladder cancer.
References
1. Cancer Research UK. Bladder cancer. 2016.
https://www.cancerresearchuk.org/
about-cancer/bladder-cancer
2. Witjes JA, Bruins HM, Carrión A, et al. EAU Guidelines on Muscle-invasive and metastatic Bladder Cancer. 2022.
https://d56bochluxqnz.cloudfront.net/
documents/full-guideline/EAU-Guidelines
-on-Muscle-Invasive-And-Metastatic
-Bladder-Cancer-2022.pdf
3. Panebianco V, Narumi Y, Altun E, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System). European Urology 2018;74(3):294‑306.
4. Bryan RT, Liu W, Pirrie SJ, et al. Comparing an imaging-guided pathway with the standard pathway for staging muscle-invasive bladder cancer: preliminary data from the BladderPath study. European Urology 2021;80(1):12‑15.
5. Yoshida S, Koga F, Kobayashi S, et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. International Journal of Radiation Oncology Biology Physics 2012;83(1):e21-7.
6. Necchi A, Raggi D, Gallina A, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. European Urology 2020;77(4):439-46.
7. Necchi A, Bandini M, Calareso G, et al. Multiparametric magnetic resonance imaging as a noninvasive assessment of tumor response to neoadjuvant pembrolizumab in muscle-invasive bladder cancer: preliminary findings from the PURE-01 study. European Urology 2020;77(5):636-43.
8. NICE. Bladder cancer: diagnosis and management. 2015.
https://www.nice.org.uk/guidance/ng2
9. Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. The Oncologist 2016;21(6):708-15.
10. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. Journal of Clinical Oncology 2014;32(18):1895.
11. Patel VG, Oh WK, Galsky MD. Treatment of muscle‐invasive and advanced bladder cancer in 2020. CA: a Cancer Journal for Clinicians 2020;70(5):404-23.
12. Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle‐invasive bladder cancer. Cancer 2015;121(15):2586-93.
13. ECOG-ACRIN Cancer Research Group. 2022.
https://ecog-acrin.org
14. Syn NL, Teng MW, Mok TS, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. The Lancet Oncology 2017;18(12):e731-41.
15. Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. Journal of Clinical Oncology 2018;36(34):3353-60.
16. Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. New England Journal of Medicine 2021;384(22):2102-14.
17. Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. The Lancet Oncology 2021;22(4):525-37.
18. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. Journal of Clinical Oncology 2019;37(29):2592.
19. Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of fibroblast growth factor receptors in carcinogenesis deregulation of FGF receptor signaling in cancer. Molecular Cancer Research 2010;8(11):1439-52.
20. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. New England Journal of Medicine 2019;381(4):338-48.
21. Pal SK, Rosenberg JE, Hoffman-Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1–3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations BGJ398 in metastatic urothelial cancer. Cancer Discovery 2018;8(7):812-21.
22. Lalondrelle S, Huddart R, Warren-Oseni K, et al. Adaptive-predictive organ localization using cone-beam computed tomography for improved accuracy in external beam radiotherapy for bladder cancer. International Journal of Radiation Oncology Biology Physics 2011;79(3):705-12.
23. Hafeez S, Webster A, Hansen VN, et al. Protocol for tumour-focused dose-escalated adaptive radiotherapy for the radical treatment of bladder cancer in a multicentre phase II randomised controlled trial (RAIDER): radiotherapy planning and delivery guidance. BMJ Open 2020;10(12):e041005.
All websites last accessed 21 July 2022.
Declaration of competing interests: None declared.
Acknowledgement: The author would like to thank Dr Darren Leaning, Consultant Clinical Oncologist, for his advice and insights.