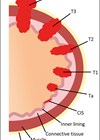
Every year, roughly 10,300 individuals are diagnosed with bladder cancer in the UK, making it the 11th most common cancer in the UK, and the eighth most common cancer in men [1]. Of those diagnosed with the disease, 75-85% will have non-muscle invasive bladder cancer (NMIBC), which is confined to the mucosa (Ta) or submucosa (T1) [2], as shown in Figure 1. In this article, we will explore recent innovations in diagnosis and treatment of NMIBC, and highlight promising future developments.
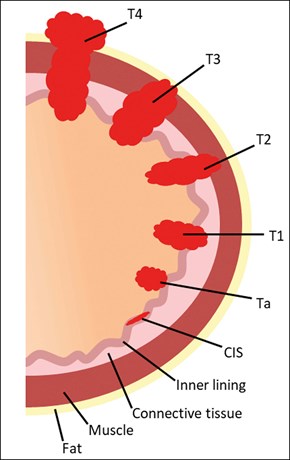
Figure 1: Bladder cancer staging.
Diagnosis
Cystoscopy
White light cystoscopy (WLC) remains the mainstay of bladder cancer diagnosis [2]. However, WLC can miss some tumours, particularly the flat lesions of carcinoma in situ (CIS). A recent prospective study demonstrated that WLC missed up to 8% of bladder cancer diagnoses [3].
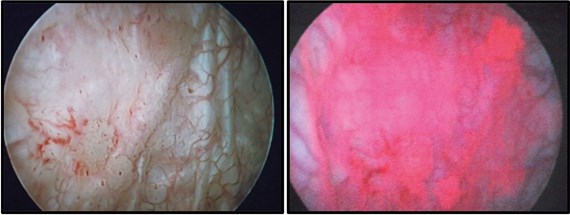
Figure 2: Cystoscopy of a patient diagnosed with CIS. The right shows the red appearance of CIS with PDD,
whilst the left shows the difficulty of detection with WLC (images courtesy of Miss J Cresswell).
Photodynamic diagnosis (PDD) involves instillation of hexaminolevulinate (Hexvix®) into the bladder. Tumour cells have a higher uptake than the surrounding mucosa, and the photoactive porphyrin emits a red fluorescence when viewed under blue light. Figure 2 demonstrates the stark difference in the appearance of CIS when using PDD versus WLC. Many studies have demonstrated the superior detection rate of cancer with PDD, however, a recent UK randomised controlled trial (PHOTO) of WLC and transurethral resection of bladder tumour (TURBT) versus PDD and TURBT, failed to show improved oncological or economic benefit of PDD [4,5].
A different technique, narrow band imaging (NBI), divides white light into two bandwidths which better reveals the hyper-vascularised tissue found in cancer, making both detection and TURBT easier. NBI guided TURBT has shown reduced recurrence rates in patients with low-risk disease specifically, but no difference versus WLC in intermediate / high-risk patients [6]. The use of NBI is routinely integrated into many flexible cystoscopy systems and has the added benefit of not requiring a prior instillation.
What the future may hold
Optical coherence tomography (OCT) provides the operator with real-time tissue architecture without the need for biopsy. Within urology, and specifically the diagnosis of bladder cancer, OCT could possibly provide real-time grade and stage of tumour at cystoscopy without the need for biopsy, but evidence is currently limited and the lack of a forward-facing OCT probe may hamper its further use and development [7].Along the same vein of providing immediate pathological diagnosis, Raman molecular imaging is a technology that, through the use of a fibre optic catheter, has been shown to have a high specificity and sensitivity in the diagnosis of suspected bladder cancer [8].
Despite this, whilst the ability to determine a lesion’s grade or stage during TURBT is useful, it is known that experienced cystoscopists possess the ability to accurately predict grade and stage from cystoscopic appearances [9].
Imaging
The role of imaging has been to detect upper tract tumours and to evaluate metastatic disease in muscle invasive bladder cancer. Albeit ultrasound and CT scans are able to detect bladder tumours, they are not yet able to replace cystoscopy [2]. CT urography can be used prior to TURBT if muscle invasion is suspected, to assess lymph nodes, and evaluate whether distant metastases are present [2]. There is recent interest in the use of a multi-parametric MRI (mpMRI) to stage bladder cancer, and a new system has been developed in an attempt to standardise bladder cancer mpMRI reports, the Vesical Imaging-Reporting and Data System (VI-RADS) [10].
The use of mpMRI and VI-RADS scoring is most accurate in patients prior to undergoing TURBT, as surgery can produce artefact. Akin to PI-RADS, used to standardise prostate mpMRI reports, VI-RADS would assign patients a score from one to five, with low scores deeming muscle invasion unlikely, and vice-versa with high scores. The development of VI-RADS has the exciting potential to streamline definitive treatment [10] by distinguishing NMIBC from MIBC. Radical treatment could be instigated with mpMRI and biopsy only, without the need for formal TURBT. This could have the effect of considerably reducing the time delay to definitive treatment.
Urinary cytology
Cytology has a high sensitivity for high-grade disease, particularly CIS, but it is unhelpful in low-grade disease. At present, it is used in conjunction with cystoscopy in the follow-up surveillance setting for patients with high-grade disease. A standardised reporting system has increased its utility [11].
Urinary molecular markers
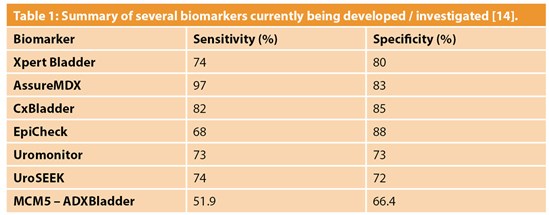
Whilst some molecular bladder cancer markers have greater sensitivity compared to urine cytology, they do not yet possess the accuracy and reliability to replace cystoscopy for diagnosis of bladder cancer. A possible role for urinary molecular markers lies in surveillance of patients with previous bladder cancer, in a hope to reduce the number of invasive cystoscopies. The burden of surveillance for NMIBC is high and negatively impacts patients’ quality of life [12]. Table 1 shows a summary of several biomarkers currently being investigated and developed. The DETECT I and DETECT II studies are aiming to prove that the UroMark assay can be used to rule out bladder cancer in patients with haematuria, and to assess whether the marker can be used to detect bladder cancer recurrence respectively [13].
Risk stratification for diagnosis
Screening programmes for bladder cancer have been considered, but none have yet satisfied the required criteria [15], though a number of international guidelines have recently been updated to focus on higher risk groups [16]. The IDENTIFY study was a prospective observational study that evaluated the prevalence of urinary tract cancer in patients referred to secondary services with visible haematuria. Their findings potentially pave the way for the development of a cancer prediction model to risk-stratify patients in a hope to reduce unnecessary over-investigation [17].
TURBT
Having diagnosed a bladder tumour, current pathways proceed to TURBT. Previously inappropriately considered definitive treatment for bladder cancer, TURBT is now primarily a biopsy used to stage and grade disease and can form part of multimodality treatment for some patients. It is only considered definitive treatment in patients with low-risk NMIBC [18]. Future management paradigms should consider TURBT as equivalent to a prostate biopsy.
Following TURBT, a number of features can predict recurrence and progression rates. Both the European Organisation for Research and Treatment of Cancer (EORTC) and the European Association of Urology (EAU) have calculators that can aid in predicting the risk of progression (the EORTC calculator can also predict recurrence), and these tools can be used to support patients in deciding on further management [2].
Regardless of the model used, as mentioned prior, TURBT quality is paramount to determine grade and stage and to contribute to effective treatment. It has been demonstrated that an absence of detrusor muscle in a TURBT specimen carries an increased risk of residual disease, earlier recurrence, and under-staging of the tumour [19]. For high-risk NMIBC, the presence of detrusor muscle in the specimen is an important factor when considering the need for re-resection for staging [20].
Treatment
As discussed above, good quality TURBT can be considered definitive treatment for low-risk NMIBC. However, due to the risk of recurrence, adjuvant therapy is considered for all intermediate and high-risk patients. Recurrence may be due to incomplete resection of the initial lesion, but it is also believed to occur due to reimplantation of cancerous cells or new tumours developing due to field change. There are several intravesical agents that are used as single postoperative instillations after TURBT, but evidence strongly advocates that regardless of the agent used, the treatment should be given within the first few hours of TURBT before the cells become implanted and are covered by extracellular matrix [2].
Intravesical chemotherapy
The use of a chemotherapy agent post-TURBT destroys circulating tumour cells within the bladder, and has an ablative effect on not only the cancerous cells at the TURBT site, but also on small, overlooked tumours that may have been missed during the procedure [2].
For patients with low-risk disease, a single immediate instillation of a chemotherapy agent is adequate. Commonly used agents include mitomycin C (MMC), epirubicin and pirarubicin, all of which have been shown to provide benefit [2].
The use of chemotherapy agents has remained largely unchanged over the years, but a recent development is the emerging evidence of effectiveness of device-assisted treatment such as electromotive drug administration (EMDA) and chemohyperthermia (CHT). Such systems include the use of a catheter system utilising either radiofrequency energy to heat the bladder wall (Synergo™), or directly heating the chemotherapy instillation (HIVEC™). A randomised controlled trial from 2016, examining the efficacy of MMC chemohyperthermia, showed increased recurrence-free survival at two years when compared to intravesical bacillus Calmette-Guérin (BCG) [21]. A further trend is the instillation of chemotherapy in the operating theatre post-TURBT, ensuring immediate treatment and facilitating the delivery of day case TURBT [22].
Intravesical Bacillus Calmette-Guérin
BCG immunotherapy has been used for decades and is thought to provoke an immune response within the bladder that prevents cancerous cell implantation / development [23]. The treatment regime involves an initial course of six doses of intravesical BCG, and maintenance treatment thereafter. The duration of maintenance treatment is determined according to the risk profile of the cancer. For example, CIS is ideally treated with up to three years of maintenance treatments, whereas intermediate-risk NMIBC is adequately treated with one year of maintenance [24].
“TURBT quality has always been, and remains, arguably the most important step in the management of these patients”
There is a host of evidence that confirms BCG post-TURBT is more efficacious at reducing the risk of NMIBC recurrence, than TURBT alone or TURBT plus intravesical chemotherapy [2]. A drawback of BCG, however, is that it has a greater side-effect profile than the chemotherapy agents, with local side-effects including cystitis and haematuria, and systemic side-effects including fever, malaise, and BCG sepsis [2].
BCG failure describes NMIBC which recurs or persists despite BCG treatment, or when BCG treatment is not tolerated due to its side-effects. There is considerable interest in development of emerging therapies to treat this group. Options being studied include novel intravesical chemotherapy agents, and immune checkpoint inhibitors [25]. Over recent years there have also been worldwide shortages of BCG, and this has further catalysed the work to find an alternative treatment [26].
Very high-risk NMIBC, such as T1G3 disease with CIS, carries a significant risk of progression to muscle-invasive disease and associated mortality [27]. Fit patients affected by such disease may opt for primary radical cystectomy, as may those with high-risk NMIBC who have failed to respond to BCG.
Conclusion
In summary, much has changed over recent years with regards to diagnosis and management of patients with NMIBC. New cystoscopic technologies hope to improve cancer detection rates and TURBT quality, and standardised mpMRI reporting seeks to streamline the process to definitive treatment of invasive disease. Alongside this, the search for a biomarker that can ideally be used for the follow-up of those treated for NMIBC continues and the development of a risk-stratification model for haematuria investigation, are both aiming to ease the burden of diagnosis and follow-up to patients.
TURBT quality has always been, and remains, arguably the most important step in the management of these patients. New agents and systems for adjuvant treatment hope to help maintain remission, especially due to the recurrent shortages of intravesical BCG.
References
1. Cancerresearchuk.org. (2019). Bladder cancer | Cancer Research UK. 2016
https://www.cancerresearchuk.org/
about-cancer/bladder-cancer
2. Babjuk M, Burger M, Compérat E, et al. EAU Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS). 2022
https://uroweb.org/guidelines/
non-muscle-invasive-bladder-cancer
3. Daneshmand S, Bazargani ST, Bivalacqua TJ, et al. Blue light cystoscopy for the diagnosis of bladder cancer: Results from the US prospective multicenter registry. Urologic Oncology: Seminars and Original Investigations 2018;36(8): 361.e1-e6.
4. Tandogdu Z, Lewis R, Duncan A, et al. Photodynamic versus white light-guided treatment of non-muscle invasive bladder cancer: a study protocol for a randomised trial of clinical and cost-effectiveness. BMJ Open 2019;9(9):e022268.
5. Heer R. Breaking News: PHOTO Trial. BAUS Annual Meeting, 21-23 June 2021, Virtual.
6. Naito S, Algaba F, Babjuk M, et al. and CROES Narrow Band Imaging Global Study Group. The clinical research office of the endourological society (CROES) multicentre randomised trial of narrow band imaging–assisted transurethral resection of bladder tumour (TURBT) versus conventional white light imaging–assisted TURBT in primary non–muscle-invasive bladder cancer patients: trial protocol and 1-year results. European Urology 2016;70(3):506-15.
7. Freund JE, Buijs M, Savci-Heijink CD, et al. Optical coherence tomography in urologic oncology: a comprehensive review. SN Comprehensive Clinical Medicine 2019;1(2):67-84.
8. Jin H, Lin T, Han P, et al. Efficacy of Raman spectroscopy in the diagnosis of bladder cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98(47):e18066.
9. During VA, Sole GM, Jha AK, et al. Prediction of histological stage based on cystoscopic appearances of newly diagnosed bladder tumours. The Annals of The Royal College of Surgeons of England 2016;98(8):547-51.
10. Panebianco V, Narumi Y, Altun E, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System). European Urology 2018;74(3):294‑306.
11. Barkan GA, Wojcik EM, Nayar R, et al. The Paris system for reporting urinary cytology: the quest to develop a standardized terminology. Acta Cytologica 2016;60(3):185-97.
12. Nayak A, Cresswell J, Mariappan P. Quality of life in patients undergoing surveillance for non-muscle invasive bladder cancer—a systematic review. Translational Andrology and Urology 2021;10(6):2737.
13. Tan WS, Feber A, Dong L, et al. DETECT I & DETECT II: a study protocol for a prospective multicentre observational study to validate the UroMark assay for the detection of bladder cancer from urinary cells. BMC Cancer 2017;17(1):1-7.
14. Sugeeta SS, Sharma A, Ng K, et al. Biomarkers in bladder cancer surveillance. Frontiers in Surgery 2021;8:735868.
15. Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. 1968; World Health Organization.
16. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. The Journal of Urology 2016;196(4):1021‑9.
17. Khadhouri S, Gallagher KM, MacKenzie KR, et al. The IDENTIFY study: the investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer – a multicentre observational study. BJU Int 2021;128:440-50.
18. National Health Service. National Cancer Waiting Times Monitoring Dataset Guidance – Version 11.0. 2020.
19. Mariappan P, Johnston A, Padovani L, et al. Enhanced quality and effectiveness of transurethral resection of bladder tumour in non–muscle-invasive bladder cancer: a multicentre real-world experience from Scotland’s quality performance indicators programme. European Urology 2020;78(4):520-30.
20. Gontero P, Sylvester R, Pisano F, et al. The impact of re-TUR on clinical outcomes in a large multi-centre cohort of T1-HG/G3 patients treated with BCG. BJU International 2016;118(1):44.
21. Arends TJ, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate-and high-risk non–muscle-invasive bladder cancer. European Urology 2016;69(6):1046-52.
22. Hodgson D, McGrath J, O’Flynn K, et al. Urology: Towards better care for patients with bladder cancer. 2022
https://www.gettingitrightfirsttime.co.uk/
wp-content/uploads/2022/01/Urology_
2022-01-12_Guidance_Bladder-cancer.pdf
23. Kates M, Matoso A, Choi W, et al. Adaptive immune resistance to intravesical BCG in non–muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clinical Cancer Research 2020;26(4):882-91.
24. Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. The Journal of Urology 2011;186(6):2158-67.
25. Packiam VT, Johnson SC, Steinberg GD. Non–muscle‐invasive bladder cancer: Intravesical treatments beyond Bacille Calmette‐Guérin. Cancer 2017;123(3):390-400.
26. Mayor N, Fankhauser C, Sangar V, Mostafid H. Management of NMIBC during BCG shortage and COVID‐19. Trends in Urology & Men’s Health 2021;12(1):7‑11.
27. Cambier S, Sylvester RJ, Collette L, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non–muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guérin. European Urology 2016;69(1):60-9.
[All links last accessed March 2022.]
Declaration of competing interests: None declared.