Patients with high-risk non-muscle invasive bladder cancer (NMIBC) that have failed Bacillus Calmette-Guérin (BCG) treatment are a difficult group to treat, and many may not be suitable for the preferred treatment option of radical cystectomy. Bladder-preserving treatments for BCG-unresponsive high-risk NMIBC can be utilised within the clinical trial setting for those unsuitable for radical cystectomy according to the most recent European Association of Urology (EAU) guidelines [1].
In January 2020, the United States Food and Drug Administration (FDA) approved the use of systemic immunotherapy with pembrolizumab in this setting. This was based on the results of the phase II Keynote-057 trial and represents the first new therapy for BCG-unresponsive NMIBC to be approved since the approval of valrubicin in 1998. The results of this trial met the primary efficacy endpoint of a complete response in 30% of patients at six months, which is higher than the previously reported complete response rates for valrubicin. Pembrolizumab is mentioned in the EAU guideline but not specially recommended by either the EAU or National Institute for Health & Care Excellence (NICE).
Within the UK alone the number of patients that progress post BCG is likely to be more than 1000 cases per year [2], and many of these patients will be elderly and have significant co-morbidities that would preclude them from radical surgical treatment. This update summarises the area of clinical need in treating BCG-unresponsive NMBIC, the mechanism of action and the current role of immune checkpoint inhibitors in treating this group of patients, in context with other available treatments.
Immune checkpoint inhibitors
Bladder tumours have one of the highest rates of mutation of all human tumours, making them a potentially good candidate for cancer immunotherapy. The greater the tumour mutation burden, the more likelihood of forming new tumour antigens through mutations being transcribed and translated. These new antigens can be identified and targeted by the immune system, making the tumours more immunogenic and improving clinical response to immunotherapy [3]. The mainstay of immunotherapy for high-risk, NMIBC has been intravesical BCG therapy since it was first shown to be effective in 1976 [4].
However, more recently, immunotherapy with inhibitors of programmed cell death protein (PD-1) and PD-1 ligand (PD-L1), together termed the immune checkpoint inhibitors, has revolutionised the treatment of metastatic bladder cancer after decades of therapeutic stagnation for this difficult-to-treat cancer. Since atezolizumab, a monoclonal IgG1 antibody that binds PD-L1, was approved in 2016 for second-line treatment of metastatic bladder cancer, several other checkpoint inhibitors have published phase II / III trial results and have obtained approval for use in both NMIBC and muscle-invasive bladder cancer (MIBC) [4-6]. Immune checkpoint inhibitors have also been approved for use in advanced melanoma, non-small-cell lung cancer and renal cell cancer, with a large number of clinical studies ongoing in urothelial cancer [6]. Pembrolizumab alone has approval for over 20 oncology indications and is projected to be the worldwide best-selling drug by 2023, with projected revenues of over $22 billion by 2025 [7].

Immunotherapy in the EAU and NICE guidelines for the treatment of bladder cancer
Table 1 summarises the current recommendations with regards to immunotherapy treatment for bladder cancer. For muscle-invasive bladder cancer, EAU guidelines state a strong recommendation to offer immunotherapy as part of a clinical trial in the neoadjuvant and adjuvant setting. The PURE-01 phase II study of neoadjuvant pembrolizumab has published results showing a good pathological response before radical cystectomy, with 37% (42/114) of patients with MIBC achieving a pT0 response and 55% (63/114) of patients achieving tumour downstaging to pT≤1 [8]. Similarly, for neoadjuvant atezolizumab before cystectomy for MIBC, the ABACUS study showed a complete pathological response rate of 29% (18/62) [9].
In metastatic bladder cancer, the 2020 EAU guidelines upgraded their recommendation, from weak to strong, for the use of pembrolizumab or atezolizumab as first-line treatment for those who are ineligible for cisplatin-based chemotherapy and PD-L1 positive. Pembrolizumab is recommended as second-line therapy by the EAU to patients progressing during, or after, platinum-based combination chemotherapy for metastatic disease. This was based on the KEYNOTE-045 randomised controlled trial that showed a significantly increased overall survival of 10.3 months in the pembrolizumab group, compared to 7.4 months in the chemotherapy group, for patients that progressed after platinum-based chemotherapy for metastatic bladder cancer [10].
In the UK NICE recommendations, atezolizumab is recommended for second-line treatment for locally advanced and metastatic urothelial cancer having previously progressed during or after platinum-based chemotherapy. On the other hand, a recent NICE appraisal concluded that pembrolizumab is not recommended in this same setting after platinum-based chemotherapy, and therefore pembrolizumab has been removed from the Cancer Drugs Fund. This was due to uncertain cost-effectiveness that did not meet the NICE acceptability criteria for end-of-life treatments. Both pembrolizumab and atezolizumab can be used within the specifications of the Cancer Drugs Fund for those patients with locally advanced or metastatic urothelial cancer who are unsuitable for cisplatin-based chemotherapy as first line therapy for metastatic disease.
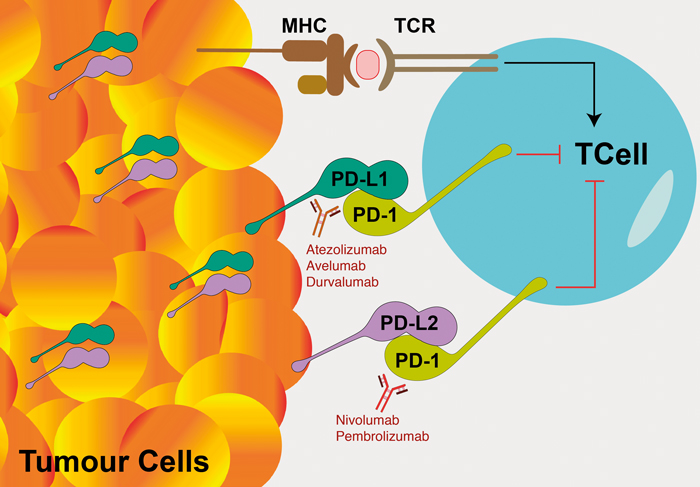
Figure 1: Diagram of the mechanism of action of checkpoint inhibitors. An anti-tumour response is triggered when intra-cellular tumour-related antigens are presented on the tumour cells’ surface in the major histocompatibility complex (MHC). These tumour antigens interact with T-cell receptors (TCR) on antigen-specific T-cells to stimulate an immune response. Tumour cells can suppress and evade immune attack by downregulation of T-cell activity. This is achieved by the expression and upregulation of programmed cell death ligands PD-L1 and PD-L2 transmembrane proteins on the tumour cells’ surface which bind to the inhibitory checkpoint molecule PD-1 receptors on activated T-cells. Checkpoint inhibitors are monoclonal antibodies which bind to PD-1 (nivolumab and pembrolizumab) and PD-L1 (atezolizumab, avelumab and durvalumab).
Mechanism of action
T-cells of the body’s immune system can kill tumour cells based on their recognition of abnormal antigens on the cancer cells’ surface (Figure 1). Checkpoint inhibitor ligands such as PD-L1 and PD-L2 are molecules created by the immune system that exists on the cell surface of healthy cells, that bind to T-cell PD-1 receptors and result in downregulation of T-cell activity, thus preventing T-cells from damaging normal tissues [24]. Cancer cells often highjack these immune checkpoint molecules to suppress and evade an immune system attack. Cancer cells display these programmed cell death ligands PD-L1 (and to a lesser extent PD-L2) on their cell surface and thus deceive T-cells to avoid apoptosis. This PD-L1 and PD-L2 interaction with PD-1 inhibits T lymphocyte survival, proliferation, functions of cytotoxicity and cytokine release and induces apoptosis of tumour-specific T-cells.
The checkpoint inhibitor ligand PD-L1 is expressed minimally by healthy tissue, but at high levels by many cancer cell types [24]. Increased PD-L1 expression has been found on immunohistochemistry with increasing bladder tumour stage and grade [6]. Immune checkpoint inhibition is a promising new area for cancer drug treatment. Several monoclonal antibodies have been developed which bind either directly to the inhibitor ligands expressed on the tumour cell surface (atezolizumab, avelumab and durvalumab bind to PD-L1) or to the checkpoint inhibitor receptors on the T cell (nivolumab and pembrolizumab bind to PD-1 (Figure 1) [24].
Expression of PD-L1
Identification of patients who are more likely to respond to immune checkpoint inhibition therapies relies on testing for expression of PD-L1 on tissue samples using immunohistochemistry. Certain checkpoint inhibitors are also restricted for use only in patients who are PD-L1 positive. However, PD-L1 expression is an imperfect biomarker, as it is known that some patients who test positive for PD-L1 may not respond to therapy, and some patients who test negative may still respond. Furthermore, the methodology and interpretation of testing for positive or negative PD-L1 status is complex and can lead to conflicting results [25]. Issues related to testing for PD-L1 expression include the significant intra-tumour heterogeneity and potential therapy-induced changes in expression [26].
To test for PD-L1 expression, the FDA, and European Medicines Agency (EMA) only recommend the use of a specifically approved PD-L1 assay for a specific checkpoint inhibitor drug. Different assays will also have different summary outputs of level of PD-L1 expression. For example, the assay approved for pembrolizumab uses an algorithm to calculate a ‘combined positive score’, which is the number of PD-L1 staining cells (including tumour cells, lymphocytes and macrophages) divided by the total number of viable tumour cells, multiplied by 100. The specimen can be considered to have PD-L1 expression if the combined positive score is one or greater. On the other hand, the algorithm for nivolumab relies on the ‘tumour proportion score’ which is percentage of total tumour cell area covered by positive tumour cells. The score for atezolizumab is based on the Ventana IC-algorithm which is scored as the proportion of tumour area covered with any discernible PL-L1 staining of any intensity in the tumour infiltrating immune cells (IC), which include lymphocytes, macrophages and cells with dendritic and reticular morphology. A specimen is considered to have PD-L1 expression if the specimen exhibits ≥1% IC, but a 5% cut-off is required to use atezolizumab as first-line therapy.
The algorithm associated with each approved assay for a checkpoint inhibitor also has different positivity cut-offs, such that inter-algorithm variability can lead to the same patient being eligible for one checkpoint inhibitor but not another. For example, a specimen may exhibit the threshold of 5% tumour infiltrating immune cells required for first-line atezolizumab therapy, but not pembrolizumab, due to an insufficient amount of additional tumour cell to immune cell ratio to reach the required combined positive score of 10 [26]. Therefore, the way PD-L1 expression is tested and reported is crucial in the decision-making process for eligibility and efficacy of use of a specific checkpoint inhibitor.
Types of checkpoint inhibitors in current use
Checkpoint inhibitors have the most evidence base in metastatic bladder cancer as second-line treatment after progression on platinum-based chemotherapy. Five checkpoint inhibitors are currently FDA approved for use in this context. Nivolumab (Opdivo®) and pembrolizumab (Keytruda®), are IgG monoclonal antibodies directed against the T-cell checkpoint inhibitor receptor programmed cell death 1 (PD-1). Atezolizumab (Tecentriq®), durvalumab (Imfinzi®) and avelumab (Bavencio®) are IgG monoclonal antibodies directed against checkpoint inhibitor ligand PD-ligand 1 (PD-L1).The regimen for these checkpoint inhibitors is IV administration every two to three weeks (up to six weekly with pembrolizumab) and they are generally better tolerated than standard systemic chemotherapy, with fewer incidences of adverse events, including serious adverse events. [27]
Adverse effects of checkpoint inhibitors
Use of checkpoint inhibitors can lead to immune-related adverse events due to excessive immune activation and inflammation of normal tissues. The tissues affected are mainly in the skin, endocrine, hepatic, and pulmonary systems. Usually these effects are mild and completely resolve on stopping the treatment and giving steroids. The general safety profile of the immune checkpoint inhibitors has been studied in a systematic review across different treatment indications.
Atezolizumab had the highest rate of adverse events, followed by nivolumab and pembrolizumab [28]. For pembrolizumab the main treatment-related adverse events were arthralgia, pneumonitis, and hepatic toxicity. For nivolumab, the toxicity spectrum was assessed to be mild and narrow, consisting mainly of endocrine toxicities. In the systematic review, nivolumab was considered the safest, especially for the treatment of lung cancer [28]. The most common side-effects of checkpoint inhibitors to warn patients about are fatigue, loss of appetite, and nausea affecting between 20% to 50% of patients. Around 10% of people or more may experience rash, and diarrhoea due to colitis. Less common adverse effects, affecting 1-10% of patients, are breathlessness and cough due to pneumonitis, subclinical hepatitis and pancreatitis which do not require treatment, kidney injury, hypothyroidism, diabetes, myocarditis, and adrenocorticotropic hormone insufficiency [29].
Current classification and recommendations for BCG-unresponsive NMIBC
There are currently no standardised treatments for BCG-unresponsive NMIBC for patients that are not suitable for radical cystectomy. This is a challenging area to treat and the EAU guideline for patients in this situation is a weak recommendation to enrol patients in clinical trials assessing new treatments or to consider bladder preserving strategies [1]. There are no NICE recommendations for this area or technology assessments of the new treatment options available [12]. NMIBC represents approximately 75% of all newly diagnosed cases of bladder cancer [30], and high-risk NMIBC accounts for approximately one third of the disease burden [31]. Generally, high-risk NMIBC comprises any of the following: a) T1 tumour, b) G3 high grade tumour, carcinoma in situ (CIS), and, c) multiple, recurrent (i.e. refractory to initial intravesical mitomycin), and large (>3cm) TaG1 / G2 low grade tumours with all three features being present [11]. After initial transurethral bladder resection and intravesical mitomycin, intravesical BCG is recommended for the treatment of intermediate and high-risk NMIBC to reduce tumour recurrence [1].
An alternative to BCG monotherapy is combination therapy of intravesical BCG plus intravesical mitomycin including using electromotive drug administration (EMDA) for delivery of MMC. This regimen showed an improved recurrence-free interval and reduced progression compared to BCG monotherapy but is not widely adopted [1,32,33]. However, up to 50% of patients with high-risk NMIBC will fail to maintain a durable response to BCG resulting in BCG failure. Approximately 50% of these failures occur in the first six months and subsequent progression to muscle-invasive disease is seen in 40% of these patients [30]. Overall, with a median follow-up of 48 to 124 months across bladder cancer trials, 21% of high-risk NMIBC cases progressed to muscle-invasive disease and 14% died, which translates into a long-term cancer specific survival of 35% in patients with high-risk NMIBC and tumour progression [34].
Different definitions of BCG failure can be categorised as: BCG-refractory, BCG-relapsing and BCG-unresponsive. BCG-unresponsive tumours are a subset of patients at higher risk of progression, who are not tumour free after BCG treatment, and for whom further BCG is not feasible and have a particularly poor prognosis.
The definition of BCG unresponsive tumours was developed in consultation with the FDA to help with the design of future clinical trials for this patient group in 2016, as at that time there was no effective therapy for BCG-unresponsive NMIBC. Table 2 describes the definitions for these different types of BCG failure.
The recommended treatment of BCG-unresponsive disease is radical cystectomy, as these tumours are unlikely to respond to further BCG therapy [1]. All other bladder-preserving strategies are considered oncologically inferior. However, it is likely that the majority of patients in this situation are not fit, or may not wish to undergo radical cystectomy. In a systemic review of bladder-sparing treatment studies in this context, 14 out of 30 included studies reported the proportion of patients undergoing cystectomy as a treatment outcome. The reported rate of cystectomy ranged widely from 5% to 58%, which is thought largely to be due to patient and clinician preference [30]. Even when comparing to a study of a group of patients aged 75 years and older with muscle-invasive bladder cancer, only 20% received radical cystectomy, 13% received chemoradiation and 66% received neither of these treatments [35]. New treatment techniques and bladder preserving strategies are given a weak recommendation by the EAU 2020 guidelines for those unable to have radical cystectomy [1].

Bladder-sparing strategies for BCG-unresponsive NMIBC
The current methods of management of these patients are not well documented and likely to be diverse in strategy [36]. Furthermore, many patients may not have had an adequate course of BCG to fully meet the definition of being BCG-unresponsive. Bladder-sparing strategies reported in use include re-induction of BCG, including with other intravesical agents such as a combination of BCG and interferon-alpha (BCG / IFN); novel delivery methods involving hyperthermia and electronic stimulation to increase the efficacy of intravesical chemotherapy (such as mitomycin C); use of intravesical chemotherapy other than mitomycin C, such as gemcitabine, valrubicin, epirubicin and docetaxel; and in some cases, systemic therapy [30,36,37].
Until recently, valrubicin (an anthracycline DNA topoisomerase II inhibitor) was the only FDA approved intravesical therapy for this context, and the indication is specifically for patients with BCG-refractory carcinoma-in-situ disease. However, valrubicin is not widely used, as response rates are suboptimal and not durable, and it is not discussed in the EAU or NICE guidelines. The approval of valrubicin was based on a phase II, multicentre, single arm trial which demonstrated a complete response rate of 16% at 12 months and 8% at 30 months [37]. Complete response (CR) is recommended by the FDA as the primary efficacy endpoint in patients with CIS at the initiation of therapy, with follow-up stipulated for every three months for the first two years and every six months for two years, and annually thereafter [30].
Other novel therapies for this patient group include enhanced delivery of intravesical mitomycin using microwave induced hyperthermia or electrically stimulated delivery, intravesical immunotherapy, systemic immunotherapy, and gene therapy [4,30,36]. Of these therapies, NICE has provided interventional procedures guidance for two novel treatments: microwave hyperthermia, and electrically stimulated intravesical chemotherapy, with both cautioned for use in the context of research only as evidence of efficacy was limited [38,39]. These novel therapies may be useful when treatment with intravesical BCG is contraindicated, or unsuitable, or has been unsuccessful.
No major safety concerns were reported for electrically-stimulated intravesical chemotherapy which consists of stimulating an electrical field across the bladder wall using cutaneous electrodes on the lower abdomen and intravesical electrodes in the mitomycin solution with the aim of increasing absorption of the drug into the bladder lining [38]. However, for intravesical microwave hyperthermia, NICE did state that there are well-recognised adverse events due to bladder thermal damage [39]. This procedure involves inserting a balloon catheter which contains a radiofrequency antenna inside the bladder. The radiofrequency antenna gives off microwaves which heat the superficial layers of the bladder wall. Mitomycin is then circulated into the bladder between the balloon surface and the bladder wall. This intravesical hyperthermia is thought to exert a direct and immune-mediated cytotoxic effect on tumour cells and improve the efficacy of chemotherapy drugs [39].
Pembrolizumab for BCG-unresponsive NMIBC: KEYNOTE-057 trial results
Since valrubicin was approved in 1998, no new therapy for BCG-unresponsive NMIBC has been approved until now. In January 2020, the FDA approved pembrolizumab as systemic immunotherapy for the treatment of high-risk NMIBC unresponsive to BCG based on phase II results of 97 patients enrolled from the KEYNOTE-057 trial [35]. Patients in the KEYNOTE-057 trial had had a median number of 12 BCG instillations, with 11.8% of patients diagnosed as CIS with T1, 24.5% as CIS with high-grade Ta and 63.7% as CIS alone. With regards for the reason for not undergoing radical cystectomy, patient refusal was the given reason in 95% of cases. Patients received pembrolizumab 200mg IV every three weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC or progressive disease or up to 24 months of therapy without disease progression. The median duration of follow-up was 15.8 months [13].
Ninety-seven patients out of 148 enrolled were included in the FDA efficacy analysis. The main primary efficacy finding was that 41% (40/97) of patients had a complete response by three months (defined by negative results at cystoscopy +/- bladder biopsy or resection, urine cytology and CT urogram imaging). Thirty percent still had a complete response by six months. The secondary efficacy endpoint was durability of response. Of these 40 patients, the median length of CR was 16.2 months. Forty percent of patients with a CR remained in CR for at least one year, which equates to 17% of the 97 patients in the primary efficacy population with durable CR of ≥ one year from the time of CR [35]. At an updated median follow-up of 21.1 months (range, 4.6 to 33.4 months) treatment was ongoing in 11 patients. Eighty-eight patients had discontinued pembrolizumab due to reasons of: persistent disease (n=40), recurrent disease (n=30), adverse events (n=10), achieved CR (n=2) and other protocol reasons (n=3) [35].
No patients developed muscle invasive or metastatic disease. In terms of tolerability, treatment-related adverse events occurred in 64.7% of patients, of which the most frequent were pruritis (11%), diarrhoea (11%), fatigue (10%). Grade 3/4 treatment-related adverse events occurred in 13% of patients and immune-mediated adverse events occurred in 19% of patients [13]. Grade 3/4 cases of hyponatremia and arthralgia was observed in three and two patients, respectively. There was one case each of grade 3/4 adrenal insufficiency, severe skin reaction, and type 1 diabetes mellitus.
Comparison of pembrolizumab to current intravesical and systemic therapies for BCG-unresponsive NMIBC
As part of the FDA review of the evidence for approval of pembrolizumab in this context, a literature review and meta-analysis was performed of existing treatments available in the United States with reported complete response rate with CIS, with or without papillary tumours. Of the 28 studies identified, only four met the criteria for inclusion in this analysis which included single arm trials for valrubicin (two studies), docetaxel and nab-paclitaxel. The complete response rate for valrubicin at six months was 18% in both valrubicin studies, with a median duration of response of 13.5 months. Overall, there was a pooled complete response rate of 21% for 197 patients included in the analysis. Outside of this review, other studies reporting on intravesical chemotherapy treatments including combination of gemcitabine and docetaxel, nab-paclitaxel and sequential gemcitabine plus mitomycin have shown complete response rates ranging from 30% to 50% at one to three year follow-up, but these are usually single-institution, non-randomised, retrospective studies [40].
Therefore, based on these results, the FDA has suggested that an investigation agent for this group of patients should demonstrate a 40-50% CR rate at six months for BCG-refractory CIS and recurrence free-survival rate for CIS/Ta/T1 tumours of 30% at 18-24 months with the lower limit of the 95% confidence interval excluding 20%. In the KEYNOTE-057 study, the lower bound of the 95% CI for the primary endpoint of CR rate was greater than the historical control CR rate of 20%; therefore, the study results for pembrolizumab monotherapy met the prespecified success criterion [35].
Current trials of immunotherapy for BCG-unresponsive NMIBC
Evidence is also awaited from the other (at least) 13 currently open trials of immunotherapy for BCG-unresponsive NMIBC patients, trialling pembrolizumab, atezolizumab, nivolumab, durvalumab, and ALT-803 (a recombinant IL-15 complex), including both intravesical or intravenous routes, and either as monotherapy or with BCG [30,36,40]. The approval requirement for pembrolizumab specified that a single-arm trial would be adequate, given that it would be likely unethical to compare to placebo, radical cystectomy, or other investigational treatments which are not standard of care. However, of the currently open studies into immunotherapies for NMIBC, there are currently several with a comparator group of BCG or external beam radiotherapy [4].
Immunotherapy may also have a role to play at an earlier stage in the treatment course for high-risk NMIBC. This can potentially reduce the risk of BCG resistance and increase the anti-tumour activity of BCG used with combination treatment of BCG and immunotherapy. There is a phase III trial (KEYNOTE-676), currently recruiting, which aims to randomise over 500 patients by 2022 with persistent or recurrent high-risk NMIBC after BCG induction therapy. This will compare either continuation of BCG therapy alone (for up to three years), or treatment with BCG plus systemic pembrolizumab, for up to two years [41,42]. Studies of combination treatment with immunotherapy may also be utilised in the future, with use of newer molecules, in both the BCG-naïve and the BCG-unresponsive setting. These include antibody-drug conjugates such as enfortumab vedotin and sacituzumab govitecan, as well as vaccines, immunomodulatory drugs, and oncolytic viruses [30,43-46]. There is a wealth of new opportunities on the horizon that hopefully will make NMIBC a more treatable condition, with the opportunity to offer patients bladder preservation with more confidence than we currently have.
Conclusion
Defining the patient population for the purposes of conducting clinical trials into effective treatment for BCG-unresponsive NMIBC is difficult but has been more standardised since the classification of BCG failure was updated in 2016. It can be seen that comparing efficacy across treatments for this patient group is difficult due to the requirements to meet the criteria for BCG-unresponsive disease as well as reporting a standardised efficacy endpoint. Given that the NHS indicative price for pembrolizumab (Keytruda®) is currently £2360 per 100mg, it would cost £165,200 for a full 35 cycles of treatment as described in the KEYNOTE-057 protocol. In December 2019, NICE decided to not recommend pembrolizumab for use as second-line therapy for locally advanced and metastatic urothelial cancer as the cost-effectiveness estimate is above the threshold NICE considers acceptable for end-of-life treatments [21].
Although approved by the FDA, it remains to be seen whether uptake will be any higher than for valrubicin in the United States, and whether, unlike valrubicin, it will be recommended by guideline bodies. Immunotherapy is recommended within the EAU and NICE guidelines for certain subsets of locally advanced and metastatic urothelial cancer, and it is likely further approvals and guideline recommendations will include the use of immunotherapy as a treatment option, either as monotherapy or in combination with other treatments, for the treatment of NMIBC.
References
1. Babjuk M, Burger M, Compérat E, et al. EAU Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS). Arnhem, The Netherlands: EAU Guidelines Office; 2020.
https://uroweb.org/guideline/
non-muscle-invasive-bladder-cancer/.
2. UK Department of Health. Unavailability of Intravesical BCG for Treatment of Bladder Cancer. 2012.
https://assets.publishing.service.gov.uk/
government/uploads/system/uploads/attachment
_data/file/212839/bladder-cancer-letter.pdf.
3. Wu Z, Wang M, Liu Q, et al. Identification of gene expression profiles and immune cell infiltration signatures between low and high tumor mutation burden groups in bladder cancer. Int J Med Sci 2020;17(1):89-96.
4. Chehroudi AC, Black PC. Emerging intravesical therapies for the management of bacillus Calmette–Guérin (BCG)-unresponsive non-muscle-invasive bladder cancer: Charting a path forward. Can Urol Assoc J 2020;14(6).
5. Lenfant L, Aminsharifi A, Seisen T, Rouprêt M. Current status and future directions of the use of novel immunotherapeutic agents in bladder cancer. Curr Opin Urol 2020;30(3):428-40.
https://journals.lww.com/co-urology/
Fulltext/publishahead/Current_status_and_future
_directions_of_the_use_of.98998.aspx.
6. Donin NM, Lenis AT, Holden S, et al. Immunotherapy for the Treatment of Urothelial Carcinoma. J Urol 2017;197(1):14-22.
7. Smith A. Keytruda to be ‘top drug’ by 2023. Pharma Times 2019.
http://www.pharmatimes.com/news/
keytruda_to_be_top_drug_by_2023_1312064.
Accessed 9 April 2020.
8. Necchi A, Raggi D, Gallina A, et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol 2020;77(4):439-46.
9. Powles T, Rodriguez-Vida A, Duran I, et al. A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). J Clin Oncol 2018;36(15_suppl):4506.
10. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-26.
11. Witjes J, Bruins H, Cathomas R, et al. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Arnhem, The Netherlands: EAU Guidelines Office; 2020.
https://uroweb.org/guideline/
bladder-cancer-muscle-invasive-and-metastatic/.
12. National Institute for Health & Care Excellence. NICE guideline NG2. Bladder cancer: diagnosis and management. 2015.
13. De Wit R, Kulkarni GS, Uchio EM, et al. Pembrolizumab (pembro) for patients (pts) with high-risk (HR) non–muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guérin (BCG): Updated follow-up from KEYNOTE-057. J Clin Oncol 2019;37(15_suppl):4530.
14. Apolo AB, Rosenberg JE, Kim WY, et al. Alliance A031501: Phase III randomized adjuvant study of MK-3475 (pembrolizumab) in muscle-invasive and locally advanced urothelial carcinoma (MIBC) (AMBASSADOR) versus observation. J Clin Oncol 2019;37(7_suppl):TPS504-TPS504.
15. National Institute for Health & Care Excellence. Atezolizumab for untreated PDL1-positive locally advanced or metastatic urothelial cancer when cisplatin is unsuitable. Technology appraisal guidance TA492. 2018.
https://www.nice.org.uk/guidance/ta492/resources/
atezolizumab-for-untreated-pdl1positive-locally-advanced
-or-metastatic-urothelial-cancer-when-cisplatin
-is-unsuitable-pdf-82605083595973.
16. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389(10064):67-76.
17. National Institute for Health & Care Excellence. Atezolizumab for treating locally advanced or metastatic urothelial carcinoma after platinum-containing chemotherapy. Technology appraisal guidance TA525. 2018.
https://www.nice.org.uk/guidance/ta525/resources/
atezolizumab-for-treating-locally-advanced-or-metastatic
-urothelial-carcinoma-after-platinumcontaining
-chemotherapy-pdf-82606842153925.
18. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391(10122):748-57.
19. National Institute for Health & Care Excellence. Pembrolizumab for untreated PDL1-positive locally advanced or metastatic urothelial cancer when cisplatin is unsuitable.Technology appraisal guidance TA522. 2018.
https://www.nice.org.uk/guidance/ta522/resources/
pembrolizumab-for-untreated-pdl1positive-locally
-advanced-or-metastatic-urothelial-cancer-when
-cisplatin-is-unsuitable-pdf-82606837115077.
20. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-92.
21. National Institute for Health & Care Excellence. Final appraisal document – Pembrolizumab for treating locally advanced or metastatic urothelial carcinoma after platinum-containing chemotherapy. 2020.
https://www.nice.org.uk/guidance/gid-ta10466/
documents/final-appraisal-determination-document.
22. National Institute for Health & Care Excellence. Nivolumab for treating locally advanced unresectable or metastatic urothelial cancer after platinum-containing chemotherapy. Technology appraisal guidance TA530. 2018.
https://www.nice.org.uk/guidance/ta530/
resources/nivolumab-for-treating-locally-advanced
-unresectable-or-metastatic-urothelial-cancer-after
-platinumcontaining-chemotherapy-pdf-82606894222021.
23. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18(3):312-322.
24. Doyle E, Crew J, Mostafid H, et al. Urothelial cancer: a narrative review of the role of novel immunotherapeutic agents with particular reference to the management of non-muscle-invasive disease. BJU Int 2019;123(6):947-58.
25. Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med 2016;213(13):2835-40.
26. Eckstein M, Cimadamore A, Hartmann A, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med 2019;7(22):690.
27. Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol Off J Eur Soc Med Oncol 2019;30(6):970-6.
28. Xu C, Chen Y-P, Du X-J, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 2018;363.
29. Bajwa R, Cheema A, Khan T, et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res 2019;11(4):225-36.
30. Kamat AM, Lerner SP, O’Donnell M, et al. Evidence-based Assessment of Current and Emerging Bladder-sparing Therapies for Non–muscle-invasive Bladder Cancer After Bacillus Calmette-Guerin Therapy: A Systematic Review and Meta-analysis. Eur Urol Oncol 2020;3(3):318-40.
31. Thomas F, Noon AP, Rubin N, et al. Comparative Outcomes of Primary, Recurrent, and Progressive High-risk Non–muscle-invasive Bladder Cancer. Eur Urol 2013;63(1):145-54.
32. Di Stasi SM, Giannantoni A, Giurioli A, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol 2006;7(1):43-51.
33. Logan C, Brown M, Hayne D. Intravesical therapies for bladder cancer – indications and limitations. BJU Int 2012;110(S4):12-21.
34. den Bosch S van, Witjes JA. Long-term Cancer-specific Survival in Patients with High-risk, Non–muscle-invasive Bladder Cancer and Tumour Progression: A Systematic Review. Eur Urol 2011;60(3):493-500.
35. Federal Drug Administration. Pembrolizumab-P057V01MK3475 Advisory Committee Briefing Document. 2019.
https://www.fda.gov/media/133542/download.
36. Li R, Sundi D, Zhang J, et al. Systematic Review of the Therapeutic Efficacy of Bladder-preserving Treatments for Non–muscle-invasive Bladder Cancer Following Intravesical Bacillus Calmette-Guérin. Eur Urol 2020.
37. Mukherjee N, Svatek RS, Mansour AM. Role of immunotherapy in bacillus Calmette–Guérin-unresponsive non–muscle invasive bladder cancer. Urol Oncol Semin Orig Investig 2018;36(3):103-8.
38. National Institute for Health & Care Excellence. NICE IPG638: Electrically Stimulated Intravesical Chemotherapy for Non-Muscleinvasive Bladder Cancer.; 2019.
https://www.nice.org.uk/guidance/IPG638.
39. National Institute for Health & Care Excellence. NICE IPG628: Intravesical Microwave Hyperthermia and Chemotherapy for Non-Muscle-Invasive Bladder Cancer.; 2018.
https://www.nice.org.uk/guidance/IPG628.
40. Taylor J, Becher E, Steinberg GD. Update on the guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int 2020;125(2):197-205.
41. Kamat AM, Shore ND, Hahn NM, et al. Bacillus Calmette-Guerin (BCG) with or without pembrolizumab (pembro) for high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) that is persistent or recurrent following BCG induction: Phase III KEYNOTE-676 study. J Clin Oncol 2019;37(15_suppl):TPS4591-TPS4591.
42. Kamat AM, Shore N, Hahn N, et al. KEYNOTE-676: Phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Futur Oncol 2020;16(10):507-16.
43. Rosenberg JE, O’Donnell PH, Balar A V, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2019;37(29):2592-600.
44. Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab Govitecan, a Novel Antibody–Drug Conjugate, in Patients With Metastatic Platinum-Resistant Urothelial Carcinoma. Clin Genitourin Cancer 2016;14(1):e75-e79.
45. Balar AV, Kulkarni GS, Uchio EM, et al. Keynote 057: Phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). J Clin Oncol 2019;37(7_suppl):350.
46. Siddiqui MR, Grant C, Sanford T, Agarwal PK. Current clinical trials in non–muscle invasive bladder cancer. Urol Oncol Semin Orig Investig 2017;35(8):516-27.
Declaration of competing interests: Andrew Protheroe has received research funding from Merck; travel from Bayer, Astellas, Ipsen, Eisai and EUSA Pharma; and ASCO Virtual Meeting sponsorship from MSD. He is also on advisory boards for Astellas, Janssen, Pfizer, BMS, Ipsen and Merck.
Acknowledgement: The author would like to thank Dr Eliana Taconni for use of her illustration for Figure 1.