Features
An amateur’s trek into the Urology Boot Camp
Driven by a strong desire to pursue a career in medicine, I was lucky to get an opportunity to attend the Urology Boot Camp at Leeds while still in sixth form. Although urology is a postgraduate specialty, I wanted to...
Developing a core outcome set for male fertility trials
Advances in fertility treatment since in vitro fertilisation was pioneered by Edwards and Steptoe, cumulating in the birth of Louise Brown in 1978, have been nothing short of remarkable [1]. Since then, a myriad of treatments has been developed for...
Prostate cancer in men of African heritage: understanding the risk and prognostic factors
Prostate cancer (PCa) represents a major public health concern and is recognised as one of the most common cancers worldwide, accounting for a significant proportion of cancer-related deaths. It is the second most common malignancy among men globally after lung...
The microbial syndicate: dysbiosis and origins of recurrent UTIs
Traditional dogma held that urine was sterile. However, recent molecular studies have revealed an underground microbial community, known as the urinary microbiome or ‘urobiome’ [1]. Far from being harmful, this community of microorganisms helps modulate immune responses, regulate inflammation, and...
Bridging the gap – a nurse-led UTI information and support service
Urinary tract infections (UTIs) are among the most frequently diagnosed bacterial infections in both primary and secondary care. While acute, uncomplicated cases may be managed effectively, recurrent and chronic UTIs often present a more complex challenge. For many people, these...
Artificial intelligence in bladder cancer diagnostics
Bladder cancer (BCa), ranking as the 10th most common cancer worldwide, poses a significant health burden with high morbidity and mortality [1]. Timely tumour detection and accurate evaluation are crucial for effective management, as the prognosis is dependent on the...
Missed Meyer-Weigert duplex ureter during emergency ureteric stenting for ureteric stones
The Meyer-Weigert rule is a fundamental anatomical principle in urology that describes the typical orientation of ureteral orifices in duplex kidneys. In a duplex system, the upper pole ureter usually inserts inferomedially, while the lower pole ureter inserts superolaterally. This...
International urology returns to Scotland
Founded in 1907, the SIU has established itself as the premier international professional society for urologists. As global interdependence increases, so does the relevance of the Society’s mission to enable urologists in all nations to apply the highest standards of...
Can dogs smell prostate cancer?
For centuries we have known that man’s best friend has an exceptional sense of smell. ‘Sniffer’ dogs are found in a wide range of roles, including drug and explosive detection as part of airport security, helping emergency services locate survivors...
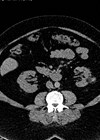
Imaging and surveillance in sporadic renal angiomyolipoma: how and when to monitor effectively
Renal angiomyolipoma (AML) are benign tumours, accounting for approximately 2–3% of all renal neoplasms [1]. Seventy percent of renal AMLs are sporadic, and 20–30% are associated with genetic aetiology. They are composed of smooth muscle, blood vessels, and adipose tissue....
Current developments and innovations of the WASHOUT study: A large-scale observational study of inpatient haematuria
Unscheduled haematuria admissions ranks among the most common urological emergencies, yet its investigation and management still lack standardisation. The readmission rate for haematuria is substantial, with reports as high as 8%, and the median hospital stay for such cases in...
Can the latest patient decision aid help OAB patients?
OAB Answers is a patient decision aid co-authored by several European urologists and gynaecologists, and two patient advocates, to help patients understand and manage their overactive bladder (OAB). It is a 36-page document split into several clear sections, aiming to...